1. Introduction
Citrus fruits are rich in bioactive substances that are valuable for disease prevention, including vitamin C, citric acid, flavonoids, carotenoids, and pectin, and they are widely consumed as fresh fruits or as processed fruit products such as juice and jam (Lee et al., 2022). Notably, citrus fruits exhibit significant pharmacological value because they contain about 60 different flavonoids known to have antioxidant, anticancer, and anti-inflammatory effects. In addition, citrus fruits contain polymethoxylated flavones (PMFs) such as nobiletin, tangeretin, and sinensetin that are not found in other fruits or vegetables (Kim et al., 2013).
In South Korea, cultivated citrus fruits currently account for approximately 30% of the total fruit production. Most are consumed as fresh fruit, and approximately 20% are used in processed fruit products. A portion of the citrus peels generated during fruit processing is dehydrated and the dried peel is used in oriental medicine for improving blood circulation and stress-related disorders (Seong et al., 2008). In addition, citrus peels are being investigated for use in the production of feeds (Joo et al., 2008) and pork patties (Choi and Lee, 2017). However, most citrus peels are still being discarded as waste, causing economic and environmental issues. Citrus peels contain various phenolic compounds, flavonoids, carotenoids, and terpenes, which can reduce the levels of low-density lipoprotein (LDL) cholesterol and increase the levels of high-density lipoprotein (HDL) cholesterol in the blood. In addition, citrus peels can facilitate capillary contraction, which can prevent hypertension (Kim et al., 2002). The flavonoids in citrus peels exhibit antioxidant, anti-inflammatory, anti-allergic, antimicrobial, anti-obesity, and immune-boosting effects (Cha and Cho, 2001; Choi et al., 2012). The total polyphenol contents (TPCs) in the pulp of well-known citrus varieties such as Kiyomi, Shiranui, Tsunokaori, and Setoka, are 6-11 mg/g, and the TPCs of the peel are 4-fold higher than those of the pulps. The antioxidant activity was also reported to be higher in the peel than in the pulp (Park et al., 2011).
South Korean citrus fruit production primarily focuses on the varieties that are harvested between November and March (Kim et al., 2009; Song et al., 1997). However, Yellowball (Citrus. hybrid cv. Yellowball), a novel citrus variety, can be harvested between mid-to-late March and May because it is a hybrid between the Kiyomi and Haruka varieties (Korea Seed and Variety Service, 2020). Yellowball pulp exhibits similar trends in sweetness and acidity at harvest (approximately 13 °Brix and 1%, respectively) to the sweetness (11.50 °Brix) and acidity (1.64%) of Satsuma mandarins at harvest (Ko et al., 1998; Korea Seed and Variety Service, 2020). Yellowball peel was included in a study of 13 citrus fruit varieties that used metabolite analyses to determine the phytochemical profiles and antioxidant activities of the fruits, and it exhibited significantly strong antioxidant activity. The main compounds in Yellowball were the flavonoid hesperidin, and PMFs such as tangeretin, sinensetin, nobiletin, and tetramethoxyflavone (Lee et al., 2022). However, the number of studies investigating the antioxidant compounds in Yellowball peel is currently insufficient.
Therefore, this study, measured the antioxidant activity of Yellowball peel and isolated and identified the main antioxidant compounds to provide a foundation for the use of Yellowball peel as an antioxidant agent.
2. Materials and methods
The citrus fruit used in this study was Yellowball (Citrus. hybrid cv. Yellowball), a novel citrus variety developed by the Citrus Research Institute (CRI) of the National Institute of Horticultural and Herbal Science at the Rural Development Administration; it is a hybrid between the Kiyomi (C. unshiu×sinensis) and Haruka (C. tamurana×natsudaidai) varieties. Fruits harvested in 2022 were obtained from the CRI. The peel samples isolated from the Yellowball fruits were freeze-dried and ground, then stored at −18℃ for subsequent TPC and antioxidant activity analyses.
Freeze-dried Yellowball peel powder (50 g) and 70% methanol (500 mL) were subjected to ultrasonic extraction for 30 min at ambient temperature, followed by filtration through a Whatman No. 2 filter paper. The residue was re-extracted twice with 500 mL portions of 70% methanol, and the collected extracts were freeze-dried. For the fractionation of antioxidant compounds, 3 g of the freeze-dried extract was redissolved in 30 mL of distilled water. Next, equal volumes of n-hexane, ethyl ether (ether), ethyl acetate (EA), butanol, and water were added for sequential liquid-liquid extraction in triplicate. The solvent was removed and the collected fractions were freeze-dried.
The TPC was measured using a partially modified Folin–Ciocalteu method (Singleton et al., 1999). A reaction solution containing a mixture of diluted peel extract (50 μL), distilled water (450 μL), Folin-Ciocalteu’s phenol reagent (250 μL, Sigma-Aldrich, St. Louis, MO, USA), and 20% Na2CO3 (1.25 mL) was left in the dark for 20 min, after which the absorbance was measured at 734 nm. A calibration curve was constructed using gallic acid (Sigma-Aldrich) and the TPC was expressed as mg gallic acid equivalent (GAE)/mg dry extract (DE).
The antioxidant activity of the peel extract was determined using 2,2-diphenyl-1-picrylhydrazyl (DPPH), 2,2’-azino-bis-(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS), ferric reducing antioxidant power (FRAP), and superoxide dismutase (SOD)-like activity assays. The DPPH assay was performed using a partially modified Blois method (1958). The peel extract (50 μL) was mixed with 0.1 mM DPPH solution (1 mL, Sigma-Aldrich), the mixture was reacted at ambient temperature for 30 min in the dark, after which the absorbance was measured at 514 nm. The ABTS assay was performed using the method of Re et al. (1999) with modifications. The peel extract (20 μL) was mixed with 0.35 mM ABTS solution (980 μL, Sigma-Aldrich), the mixture was reacted at 30℃ in the dark for 6 min, and the absorbance was subsequently measured at 734 nm. For the FRAP assay, a modified Benzie–Strain method (1996) was used. The FRAP reagent solution comprised a 10:1:1 (v/v/v) mixture of 300 mM acetate buffer, 10 mM 2,4,6-tris(2-pyridyl)-s-triazine (TPTZ, Sigma-Aldrich) in 40 mM HCl, and 20 mM FeCl3. The peel extract (100 μL) was mixed with distilled water (300 μL) and the FRAP reagent solution (3 mL), the mixture was reacted at 37℃ for 30 min, and the absorbance was subsequently measured at 593 nm. The SOD-like activity assay was performed using the method of Marklund and Marklund (1974) with modifications. A mixture of 50 μL of 50 mM Tris-HCl buffer (pH 8.5) containing 10 mM EDTA, 50 μL of 7.2 mM pyrogallol, and 50 μL of sample extract was reacted at ambient temperature for 10 min. To stop the reaction, 50 μL of 1 N HCl was added, and the absorbance was subsequently measured at 420 nm. A calibration curve was constructed using Trolox, a vitamin E analog (Sigma-Aldrich), and ascorbic acid (Sigma-Aldrich), as the references. The antioxidant activities were expressed as mg Trolox equivalent (TE)/mg DE or mg ascorbic acid equivalent (AAE)/mg DE, respectively.
The metabolites in the Yellowball peel methanol extract (YPE) were analyzed using ultra-performance liquid chromatography–quadrupole-time-of-flight mass spectrometry (UPLC-Q-TOF MS, Waters Corp., Milford, MA, USA) (Lee et al., 2022). The YPE was separated using an Acquity UPLC BEH C18 column (2.1×100 mm, 1.7 μm; Waters). The chromatographic conditions were as follows: mobile phase (A): water containing 0.1% formic acid; mobile phase (B): acetonitrile containing 0.1% formic acid; flow rate: 0.35 mL/min; and column temperature: 40°C. The Q-TOF MS was operated in positive and negative electrospray ionization (ESI) modes. The positive mode conditions were: scan range: 50-1,000 m/z; scan time: 1.0 s; capillary voltage: 3 kV; sampling cone voltage: 40 V, desolvation gas flow rate: 800 L/h; desolvation temperature: 400°C; and source temperature: 100°C. The negative mode conditions were: scan range: 50-1,000 m/z; scan time: 1.0 s; capillary voltage: 2.5 kV; sampling cone voltage: 40 V; desolvation gas flow rate: 600 L/h; desolvation temperature: 250°C; and source temperature: 100°C. Compounds were identified using the UNIFI software version 1.8.2.169 (Waters).
The high-activity antioxidant compounds in the EA, n-hexane, and ether fractions were isolated using semi-preparative high-performance liquid chromatography (HPLC, Shimadzu Corp., Kyoto, Japan) with a YMC-Triart C18 column (250 mm×10.0 mm I.D., 5 μm particle size, 120 Å pore size; YMC Co., Ltd., Kyoto, Japan). The chromatographic conditions were as follows: mobile phase (A) water; mobile phase (B): acetonitrile; flow rate: 3 mL/min; column temperature: 40°C; and injection volume: 100 μL. The gradient conditions were: 0 min, 0% B; 40 min, 30% B; 70 min, 60% B; 85 min, 60% B; and 87 min, 0% B. The isolated compounds were detected at 254 nm, and fractionation was performed at 1 min intervals. using a fraction collector (Bio-Rad, Herucules, CA, USA).
3. Results and discussion
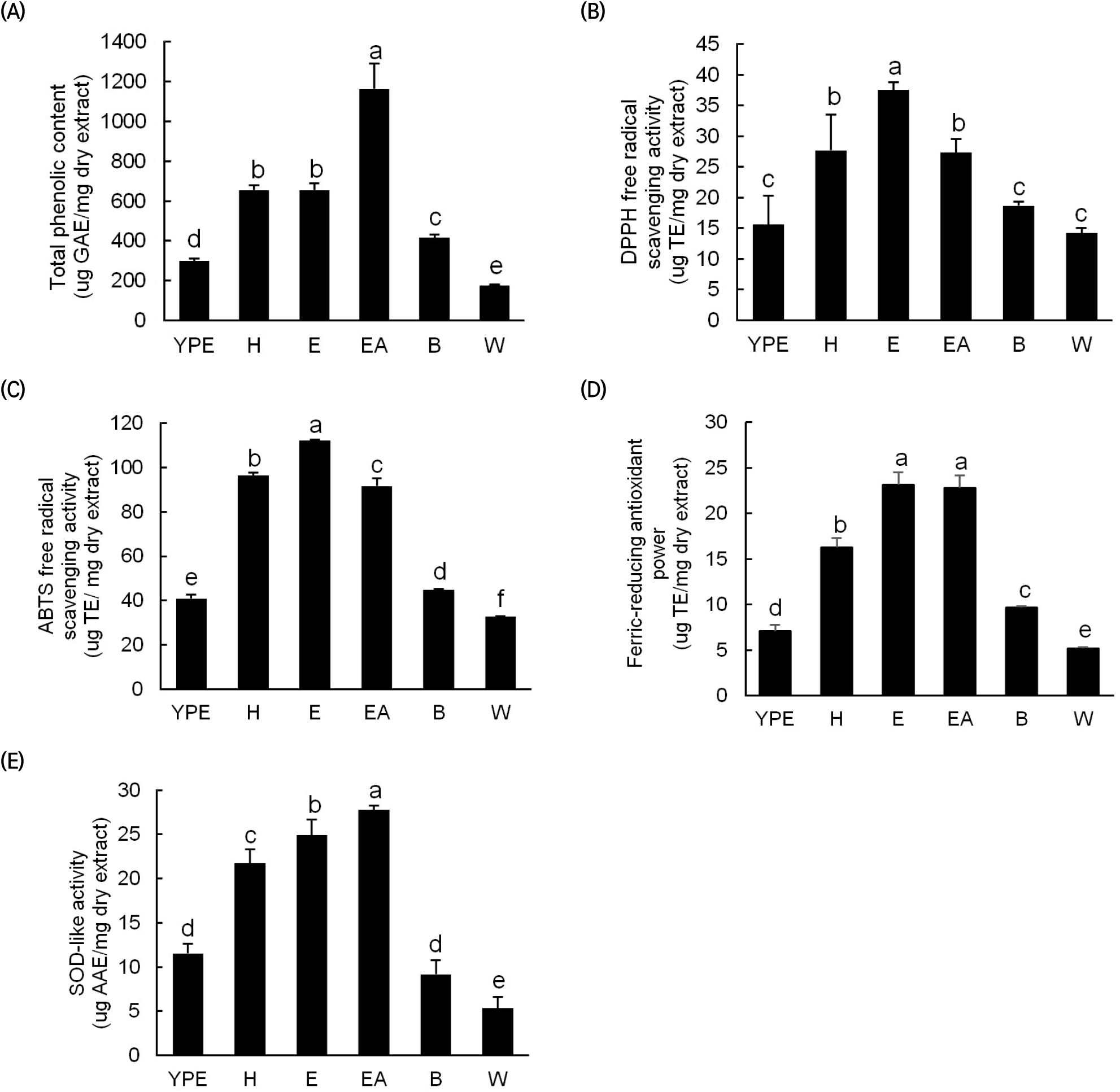
The TPC results for the YPE and its fractions (Fig. 1(A)) show that the EA, n-hexane, and ethanol fractions contain relatively high TPCs of 1,163; 655; and 654 μg GAE/mg DE, respectively. These contents are approximately 3.9, 2.2, and 2.2 fold higher than that of the YPE (298 μg GAE/mg DE). However, the TPCs of the butanol and water fractions are lower than those of the other fractions. The various antioxidant activity measurements on the YPE and fractions indicate that, as with the TPC, relatively high antioxidant activity is observed in the EA, ether, and n-hexane fractions (Fig. 1(B-E)). For these fractions, the DPPH radical scavenging activity, ABTS radical scavenging activity, FRAP, and SOD-like activity are 27-38 μg TE/mg DE, 91-112 μg TE/mg DE, 16-23 μg TE/mg DE, and 21-27 μg TE/mg DE, respectively. In contrast, the YPE and the butanol and water fractions exhibit approximately 2-fold lower activities across all measurements. Similarly, a previous study found that the DPPH radical scavenging activity of orange peel fractions was higher in EA and ether fractions than in the other fractions (Maria et al., 2006). In another previous study of Yuzu, Shiranui, and orange pulps after fractionation in various solvents, the EA fraction exhibited the strongest antioxidant activity, which was attributed to high phenolic and flavonoid contents (Assefa et al., 2016).
For the composition analysis of the YPE and its fractions, positive- and negative-mode UPLC-Q-TOF MS analyses were performed (Fig. 2). The main compounds detected in positive mode are PMFs such as sinensetin, tetramethoxyflavone, natsudaidain, tangeretin, and monohydroxy hexamethoxyflavone, which are mostly found in the n-hexane and ether fractions. In contrast, the EA fraction predominantly contains narirutin, hesperidin, cyclonatsudamine A, and natsudaidain derivatives. In the butanol and water fractions, trace amounts of natsudaidain derivatives as well as flavonoids such as saponarin, narirutin, and hesperidin, and limonoids such as zapoterin are detected. The results agreed well with those of a previous study in which citrus peel was fractionated using n-hexane, chloroform, EA, and butanol; high contents of PMFs (sinensetin, nobiletin, and tangeretin) were detected in the n-hexane and chloroform fractions, and relatively high contents of narirutin and hesperidin were found in the butanol and water fractions (Ko et al., 2010). The compounds detected in negative mode are primarily flavonoids, namely saponarin, troxerutin, narirutin, hesperidin, rhoifolin, didymin, and natsudaidain derivatives, which are also detected in positive mode. Citrus fruits, including oranges and lemons, typically contain flavonoids such as narirutin, hesperidin, and didymin, and PMFs such as saponarin that have exhibited significant efficacy in the treatment of obesity, inflammation, cancer, and cardiovascular conditions such as atherosclerosis (Choi et al., 2007; Yoon et al., 2021). Another key compound detected in the fractions is feruloyl putrescine. This phenolic amine was first isolated and identified in grapefruit leaves and juice, and is primarily found in grapefruit and oranges (Wheaton and Stewart, 1965); however, it has not been previously reported in lemons or other citrus fruits.

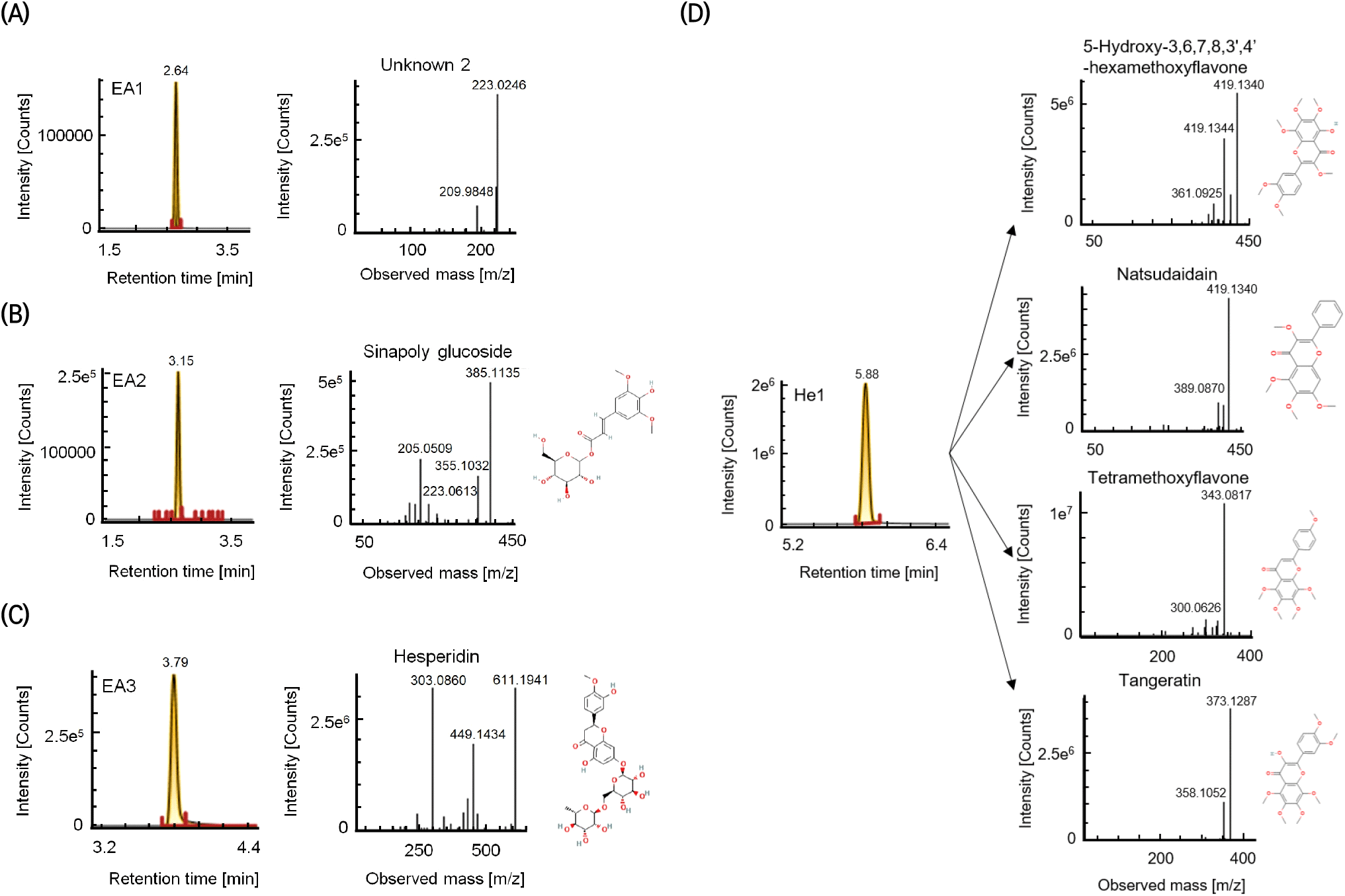
The high-activity antioxidant compounds in the EA, n-hexane, and ether fractions were isolated using HPLC with a semi-preparative C18 column. Following the isolation, the antioxidant activity of each fraction was estimated using the DPPH assay (Fig. 3). The DPPH quenching chromatogram of the EA fraction contains four peaks; those with the highest quenching activity are denoted as EA1, EA2, and EA3 based on the elution order. Although antioxidant activity peaks were detected in the n-hexane and ether fractions, only the n-hexane peak denoted as He1 exhibited relatively strong antioxidant activity and was used in subsequent analyses. The antioxidant activity of the HPLC fractions decreased in the following order: EA1 > He1 > EA2 > EA3. Notably, the EA1 and EA2 peaks in the HPLC chromatogram were relatively small compared to their strong antioxidant activities.

To identify the isolated antioxidant compounds, UPLC-Q-TOF MS was performed on the EA1, EA2, EA3, and He1 fractions from the HPLC experiment (Fig. 4). Using positive mode with a collision energy of 15-20 eV, EA2 was identified as sinapoyl glucoside (m/z 385.1135; fragments: m/z 205.0509, 355.1032, and 223.0613). Sinapoyl glucoside has been reported to exhibit strong antioxidant activity, even among sinapic acids, with antioxidant, anti-inflammatory, and anticancer activities (Kelly et al., 2008; Nićiforović and Abramovič, 2014). Using the same MS conditions, EA3 was identified as hesperidin (m/z 611.1941; fragments: m/z 303.0860 and 449.1434). He1 was identified as a mixture of PMFs, namely 5-hydroxy-3,6,7,8,3’,4’-hexamethoxyflavone (m/z 419.1342), natsudaidain (m/z 419.1340), tetramethoxyflavone (m/z 343.0817), and tangeretin (m/z 373.1287). In a previous study that correlated citrus peel metabolites and antioxidant activities, tetramethoxyflavone derivatives and tangeretin were positively correlated with the DPPH scavenging activity (Lee et al., 2021). Furthermore, 5-hydroxy-3,6,7,8,3’,4’-hexamethoxyflavone, a PMF also found in sweet orange and mandarin orange peels, has strong antioxidant and anticancer effects (Pan et al., 2007). EA1 (unknown2) exhibited the highest antioxidant activity; however, it could not be identified despite the prominent peak at m/z 223.0246. Notably, with the exception of hesperidin, the levels of these compounds were negligible in the pulp.
For comparison, the IC50 values were calculated for the DPPH scavenging activity of the high-antioxidant activity compounds isolated using HPLC, namely sinapoyl glucoside (EA2), hesperidin (EA3), unknown2 (EA1), and He1 (5-hydroxy-3,6,7,8,3’,4’-hexamethoxyflavone, natsudaidain, tetramethoxyflavone, and tangeretin) (Table 1). Unknown2 exhibits the highest antioxidant activity in this study, with an IC50 of 69.17 μg/mL, which is only slightly lower than the activity of the reference Trolox (IC50 44.86 μg/mL). In contrast, the IC50 values of sinapoyl glucoside, hesperidin, and He1 are 293.50; >2,000; and 147.16 μg/mL, which are 6.5, >44.5, and 3.3-fold higher, respectively, than that of Trolox. In addition, the natural abundance (%) of each compound was calculated by dividing the intensity their corresponding UPLC-Q-TOF MS peaks by the total peak intensity of all detected compounds in the YPE. The calculated percentages were <0.01, 0.05, 7.62, and 12.31% for unknown2, sinapoyl glucoside, hesperidin, and He1, respectively. Although only a trace amount of unknown2 was found in the Yellowball peel, it exhibited the highest antioxidant activity. Sinapoyl glucoside and unknown2, despite not being identified as the main components in this study, were confirmed to be novel antioxidant compounds that have not been reported in previous studies on the antioxidant activity of citrus fruits. These results thus provide basic data on the antioxidant compounds and support the potential use of Yellowball peel as an antioxidant agent.
Data are expressed as the mean±standard deviation of triplicate experiments. Trolox was used as a positive control. He1 compounds comprised 5-hydroxy-3,6,7,8,3’,4’-hexamethoxyflavone, natsudaidain, tetramethoxyflavone, and tangeretin.
4. Conclusions
The antioxidant effects of the peel of Yellowball, a novel citrus variety, were determined using various techniques. To isolate and identify the main antioxidant compounds, the peel extract was fractionated in different organic solvents. The EA, ether, and n-hexane fractions demonstrated high antioxidant activities, and were fractionated once more using semi-preparative HPLC to obtain four final fractions with high antioxidant activities (EA1, EA2, EA3, and He1). UPLC-Q-TOF MS was used to identify the antioxidant compounds in these fractions. EA2 was identified as sinapoyl glucoside, EA3 as hesperidin, and He1 as a PMF mixture containing 5-hydroxy-3,6,7,8,3’,4’-hexamethoxyflavone, natsudaidain, tetra-methoxyflavone, and tangeretin. EA1 contained a compound with a m/z of 223.0246 [M-H]; however, the compound could not be identified. Comparing the DPPH scavenging activity of these compounds showed that the antioxidant activity decreased in the following order: unknown2 (69.17 μg/mL) > He1 (44.86 μg/mL) > sinapoyl glucoside (293.50 μg/mL) > hesperidin (>2,000 μg/mL). Notably, in the case of unknown2, the antioxidant capacity was similar to that of Trolox, despite its low content the Yellowball peel. In addition to sinapoyl glucoside, unknown2 is a novel antioxidant compound that has not been identified in previous studies on the antioxidant activity of citrus fruits, and it was shown to be a key antioxidant compound in Yellowball peel. Further studies are needed to precisely identify the compounds and elucidate their antioxidant mechanisms; nevertheless, the findings suggest that compounds other than the previously known flavonoids contribute to the antioxidant activity of citrus fruits. The results of this study also provide a foundation for the efficient use of Yellowball peel as an antioxidant material in the future.
