1. Introduction
Recent improvements in income levels and increased social activity have led to a steady growth in the global cosmetics industry, forming a market worth approximately 351.6 billion dollars as of 2015 (Lee, 2017). With the enforcement of the “Cosmetics Act”, the cosmetics-related industry is increasingly demanding advanced and functional technologies, as well as new ingredient development. Markets related to functional cosmetics with skin-whitening and anti-aging are expected to grow rapidly. According to the recent trend of being environmentally friendly and nature-oriented, the active ingredients in cosmetics are mixed and used in various forms based on animal or plant-derived natural products. This development trend is expected to continue in the future, with a preference for natural and organic products (Sohn et al., 2004).
Melanin is a natural polymeric pigment widely found in animals and plants, and its biosynthesis is promoted as a mechanism to protect against skin damage caused by ultraviolet radiation (UVR), such as the synthesis of melanin to absorb more than a certain amount of UVR to block and protect (Park, 2015; Yoneta et al., 2004). With increasing levels of exposure to UVR, melanin can be excessively synthesized, and the physiological function of the skin decreases owing to aging, causing damage to skin connective tissue and diseases such as pigmentation in various forms such as freckles (Hill et al., 1997). The outermost layer of the human skin consists of the epidermis, dermis, and hypodermis, and the basal layer of the epidermis is directly exposed to the external environment, containing melanocytes. The production of melanin takes place in melanosomes which are produces in melanocytes. There are two major types of melanin: pheomelanins (complexes of lighter yellow and red pigments) and eumelanins (darker brown and black pigments). Generally, all types of skin have a higher proportion of eumelanin than pheomelanin (Brenner et al., 2008). The binding of α-melanocyte stimulating hormone (α-MSH) to melanocytes increases by UVR, and α-MSH is produced with several other peptides, by the proteolytic cleavage of the large precursor protein pro-opiomelanocortin (POMC) (Tsatmali et al., 2002). Melanocortin 1 receptor (MC1R) involved in regulating melanin synthesis and, is one of the key receptors. α-MSH binds to MC1R which positively regulates melanogenesis. α-MSH stimulates the production of intracellular concentration of cyclic adenosine monophosphate (cAMP) by activating adenylyl cyclase (AC). cAMP production protein kinase A (PKA) activation in turn activates microphthalmia-associated transcription factor (MITF) by phosphorylating cAMP response element-binding protein (CREB). MITF plays a fundamental role in the transcriptional regulation of melanogenesis. In this process transforming growth factor-β (TGF-β1) inhibits the activity of PKA activated by MC1R to downregulate the activity of CREB-MITF transcription factor. MITF regulates the expression of TYR, TRP-1, and TRP-2. In melanin synthesis, biochemical-catalyzed and complex enzymatic reactions are involved. Enzymatically, tyrosinase-related protein-1 (TRP-1), tyrosinase-related protein-2 (TRP-2), and tyrosinase (TYR) are key regulators of melanogenesis. Synthesis of melanin begins by the oxidation of L-dihydroxyphe-nylalanine (L-DOPA) and/or L-tyrosine to dopaquinone (DQ), which play a role as a substrate for the synthesis of eumelanin and pheomelanin (Pillaiyar et al., 2018; Slominski et al., 2004). As a result of the reactions of these enzymes and factors, the synthesized melanin accumulates in melanosomes and is transported to keratinocytes. The transportation of melanosomes to the melanocyte dendrite primarily involves three components of proteins: rab27a, melanophilin, and myosin Va (myo5a). When these three components form a tripartite protein complex, it is possible to transfer melanosomes to the dendrite tips, then melanin is transferred to keratinocytes (Fukuda et al., 2002).
Bioconversion refers to the technology of producing and manufacturing various metabolites through fermentation using microorganisms and enzymes. Unlike traditional fermentation processes, bioconversion utilizes the selectivity of microorganisms or enzymes to produce products from precursors. Bioconversion processes, which have enhanced fermentation technology, are particularly used in various ways in the fields of functional foods, modern pharmaceuticals, and cosmetics (Fuller, 1989).
Fermented cosmetics enhance the rapid absorption of active ingredients into the skin during the microbial fermentation process and improve the efficacy of existing enzymes. Fermented cosmetics are emerging as a trend in the domestic cosmetics market and several domestic major companies introducing cosmetics that utilize fermentation techniques, resulting in high sales and rapid growth (Lee et al., 2010). Various studies have been conducted to increase the biological activity and active ingredients of natural products through probiotic fermentation (Li et al., 2023).
Trigonotis radicans var. sericea, commonly known as “Chamkkotmari,” belongs to the Boraginaceae family and is a perennial herb found in various fields and moist areas in Korea. It typically grows in sunny or semi-shaded environments, reaching a height of 10-15 cm and its leaves are 1.5-4 cm long, pointed at the end, and oval. The flowering time is from May to July, with light navy flowers that are approximately diameter of 0.7-1 cm. The fruits appear around September, and the young sprouts of this plant are used as food (Jung et al., 2010).
To date, there have been no studies on the bioconversion of T. radicans through microbial fermentation. Therefore, this study induced the bioconversion of T. radicans using Lactobacillus brevis isolated from the traditional Korean fermented food, kkakdugi. Additionally, this study aimed to provide fundamental data for the potential use of fermented T. radicans extracts as natural product-based functional cosmetic ingredients by confirming the differences in whitening improvement between the extracts of non-fermented and fermented T. radicans.
2. Materials and methods
Trigonotis radicans was purchased from the Korea Native Wild Grass Laboratory (Busan, Korea). The roots were removed, whole plants were washed and dried in a dry oven (FO600M, Jeiotech, Daejeon, Korea) at 50°C. The dried plants were pulverized into 40 mesh size and stored at 4°C for use as a sample.
Lactobacillus brevis used for the fermentation of T. radicans was isolated from kkakdugi and stored at the School of Food Science and Biotechnology at Kyungpook National University. Lactobacillus brevis was preserved by mixing with a 40% glycerol (Duksan, Seoul, Korea) and culture medium ratio of 1:1 and stored at −80°C. Lactobacillus brevis was inoculated into MRS broth, subcultured twice, and 2% (v/v) of L. brevis culture medium was inoculated into 200 mL of MRS broth in a triangular flask and incubated at 37°C for 24 h.
The fermentation of T. radicans was performed by adding 40 g of T. radicans powder to 200 mL of L. brevis culture medium in an Erlenmeyer flask and incubated in a 37°C shaking incubator for 72 hours at 120 rpm. The fermented samples were freeze-dried and used as fermented T. radicans samples. Sixty percent ethanol was selected as the optimal extraction solvent for T. radicans and fermented T. radicans, as reported by Cho et al. (2022).
B16-F10 cells isolated from the skin tissue of a mouse with melanoma were purchased from ATCC and cultured in Dulbecco’s Modified Eagle’s medium (DMEM) containing 1% penicillin/streptomycin and 10% fetal bovine serum (FBS). The cells were cultured in a 5% CO2 incubator at 37°C and subcultured at a density of approximately 80% growth.
Cell viability was measured as described by Carmichael et al. (1987). B16-F10 cells were seeded at a density of 5×103 cells per well in a 48-well plate and cultured in a 5% CO2 incubator at 37°C for 24 h. Each freeze-dried powder of 60% ethanol extracts of T. radicans and fermented T. radicans was prepared for sample making, sample was added 500 μL per well in different concentrations, and the cells were cultured under the same conditions for 18 h. After adding 50 μL of 5 mg/mL MTT solution, the plate was incubated in a 5% CO2 incubator for 4 h at 37°C, the culture medium was removed, and 500 μL of DMSO was added. The absorbance was measured at 540 nm using a SPECTROstar Nano Microplate Reader (BMG Labtech). Cell viability(%) was calculated as (1 - Absorbance of sample / Absorbance of control) × 100.
The melanin contents were measured using the method described by Hosoi et al. (1985). B16-F10 cells were seeded at a density of 5×105 cells per well in 100-mm dishes for cell cultures and cultured in a 5% CO2 incubator at 37°C for 24 h. Subsequently, control and sample groups were stimulated with 1 μM α-MSH for 1 h, each freeze-dried sample of T. radicans and fermented T. radicans was treated by concentration, and incubated for 48 h in a 5% CO2 incubator at 37°C. After treatment, the cells were washed with phosphate buffer saline (PBS) and stored at −80°C. Mixture of mammalian protein extraction reagent M-PER (Thermo Fisher Scientific) and protease was added 100 μL per well and lysis at 4°C. The harvested cells were centrifuged at 12,000 rpm for 20 minutes at 4°C. The lysate was stored at −80°C until western blot analysis and collected pellet was dissolved with 1 N sodium hydroxide 10% DMSO at 70°C for 1 h using a heating block (Daihan Scientific Co., Wonju, Korea) The melanin contents were measured at 405 nm using a SPECTROstar Nano microplate reader (BMG Labtech.). Melanin content was calculated as (1 - Absorbance of sample / Absorbance of control) × 100.
The lysate obtained by centrifugation was quantified using a PierceTM Bicinchoninic Acid (BCA) Protein Assay Kit (Thermo ScientificTM), and 20 μL of protein was loaded onto 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and separated by electrophoresis at 100 V for 1.5 h. The separated proteins were transferred to polyvinylidene difluoride (PVDF) membranes for 2.5 h at 60 V using a transfer cell device, and incubated in 5% bovine serum albumin blocking buffer in Tris-Buffered Saline with Tween 20 (TBST) for 1 h. Then membranes were incubated overnight at 4°C using MITF and GAPDH antibody. The membranes were washed three times with TBST and incubated for 1 h using secondary antibodies, rabbit and mouse. Protein bands were detected using a Chemiluminescent Western Blot Imager AZURE 300 (AZURE Biosystems, Dublin, CA, USA) using West Pico PLUS Chemiluminescent Substrate (Thermo ScientificTM, Rockford, USA). Images of the detected bands were analyzed and quantified using ImageJ software version 1.51j8 (Wayne Rasband, National Institutes of Health, USA).
B16-F10 cells were seeded at a density of 5×105 cells per well in 100-mm dishes for cell cultures and cultured in a 5% CO2 incubator at 37°C for 24 h. Subsequently, control and sample groups were stimulated with 1 μM α-MSH for 1 h, each freeze-dried sample of T. radicans and fermented T. radicans was treated by concentration, and incubated for 48 h in a 5% CO2 incubator at 37°C. The cells were washed with phosphate buffer saline (PBS) and stored at −80°C. Total RNA was extracted using TRI-SolutionTM (BSK-Bio, Daegu, Korea) to compare and analyze the mRNA expression of melanogenesis-related genes. The extracted total RNA samples were stored at −80°C, and cDNA was synthesized using an UltraScript 2.0 cDNA Synthesis Kit (PCR Biosystems Ltd., London, UK). 1 μL of synthesized cDNA was diluted with 5 μL of qPCRBIO SyGreen Blue Mix Lo-ROX (PCR Biosystems Ltd.), 0.3 μL of forward and reverse primer, and 3.4 μL of nuclease-free water. The PCRmax Eco 48 Real-Time qPCR system (PCRmax, Staffordshire, UK) was used to analyze mRNA expression, RT-qPCR was performed using the primer sequences listed in Table 1 and the PCR conditions were listed in Table 2.
The experiments were repeated three times for each measurement, and the results were presented as the mean±standard deviation. Significant differences (p<0.05) were compared and analyzed using Duncan’s multiple range test and one-way analysis of variance using IBM SPSS Statistics 25 (Statistical Package for Social Science, Chicago, IL, USA).
3. Results and discussions
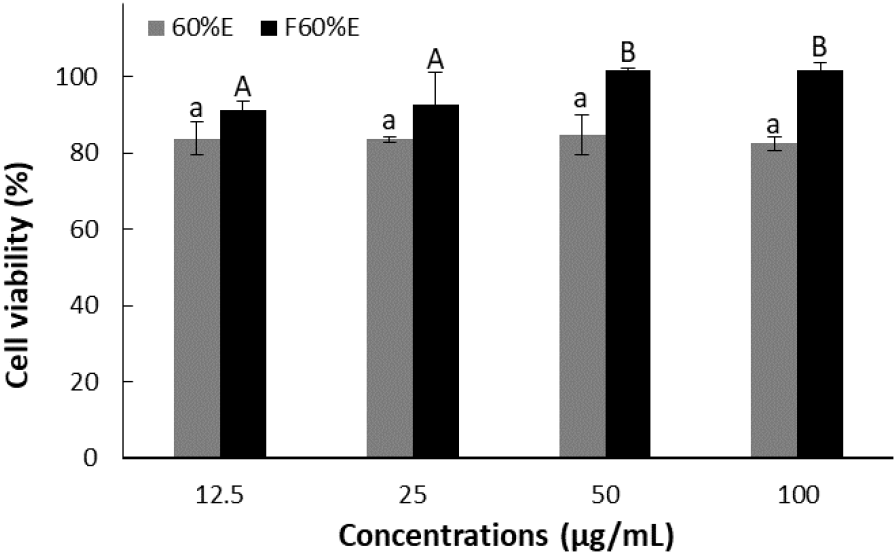
The results of cell viability of treatment with concentrations of 12.5, 25, 50, and 100 μg/mL using each freeze-dried powder from T. radicans and fermented T. radicans are shown in Fig. 1. As a result, T. radicans showed cell viability of 83.74, 83.54, 84.75, and 82.47%, while fermented T. radicans showed higher cell viability of 91.31, 92.59, 101.83, and 101.63% at 12.5, 25, 50, and 100 μg/mL. Fermented T. radicans showed significantly lower cell toxicity, confirming that bioconversion through fermentation reduced the toxicity of the extracts.
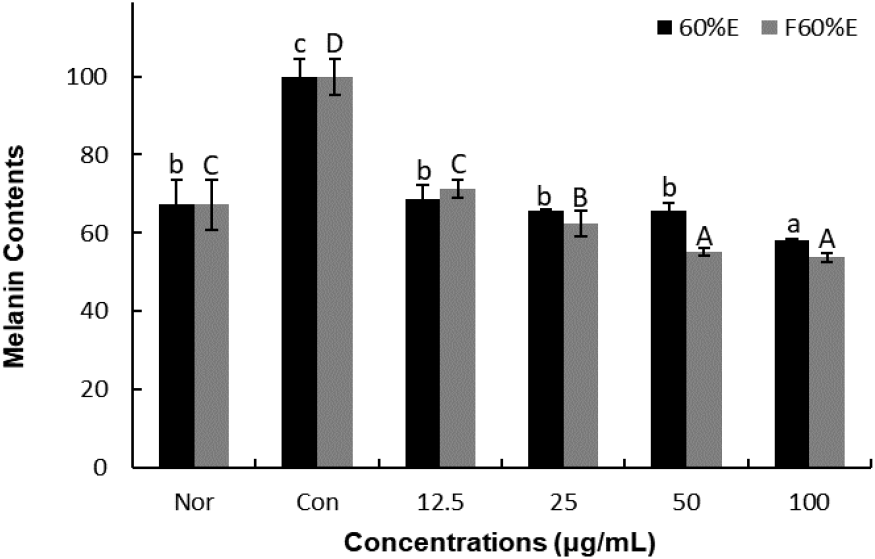
To confirm the effects on melanin contents, freeze-dried samples of T. radicans and fermented T. radicans were treated at 12.5, 25, 50, and 100 μg/mL. As shown in Fig. 2., T. radicans showed melanin contents of 68.61, 65.54, 65.65, and 58.27%, and fermented T. radicans showed 71.41, 62.36, 55.21, and 53.71% at 12.5, 25, 50, and 100 μg/mL. T. radicans and fermented T. radicans showed a concentration-dependent decrease in melanin contents. Fermented T. radicans showed similar melanin contents compared to normal group at 12.5 and 25 μg/mL and showed lower melanin contents than the normal group at 50 and 100 μg/mL. Fermented T. radicans showed lower melanin contents than T. radicans at 25, 50, and 100 μg/mL. It is confirmed that fermented T. radicans effectively reduced melanin content and showed potential as a good material for whitening functional cosmetics.
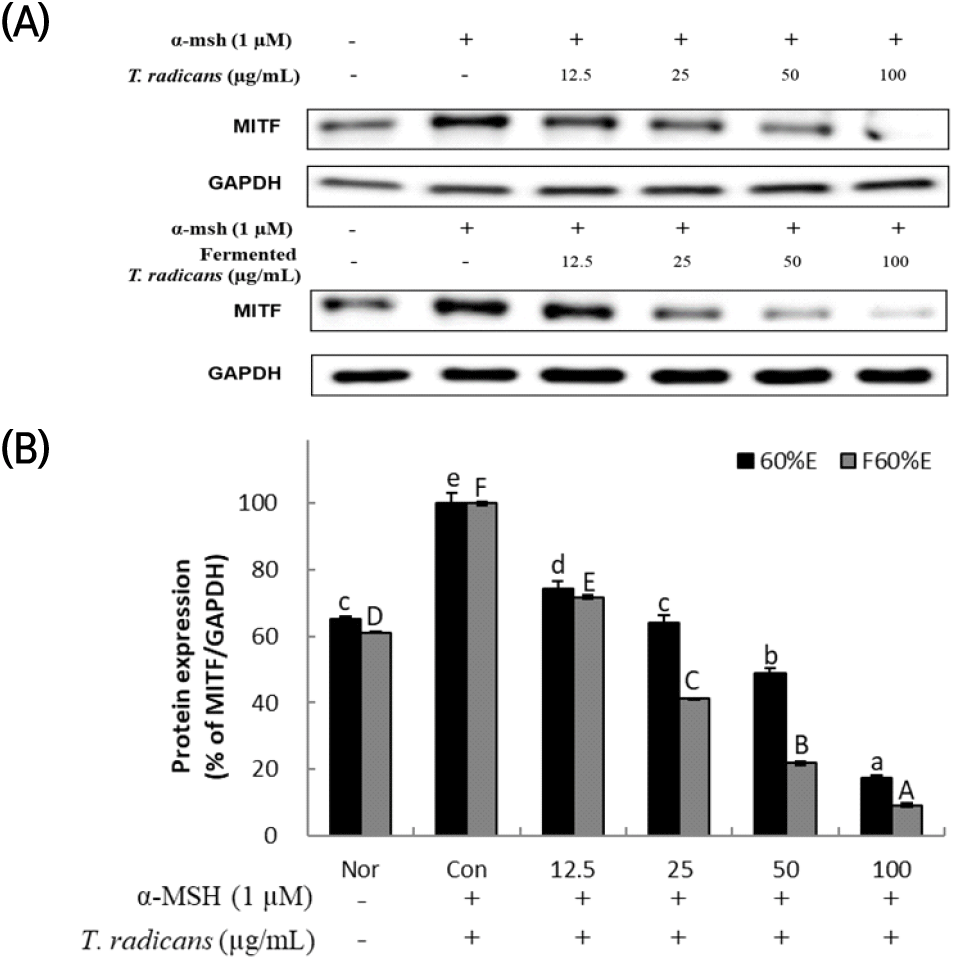
All signaling pathways are associated with a master regulator of melanogenesis MITF, which controls melanogenesis gene expression (Pillaiyar et al., 2018). After confirming that T. radicans and fermented T. radicans decreased melanin production stimulated by α-MSH, expression of MITF, a transcription factor, was analyzed by western blot to confirm the effect on melanogenesis-related protein expression. As shown in Fig. 3, T. radicans and fermented T. radicans both showed significant concentration-dependent decrease in MITF expression, T. radicans showed MITF expression of 74.49, 64.14, 48.86, and 17.42% at 12.5, 25, 50, and 100 μg/mL, and showed lower expression at 50 and 100 μg/mL than normal group. Fermented T. radicans showed 71.78, 41.20, 21.84, and 9.21% at 12.5, 25, 50, and 100 μg/mL, confirmed significant decreases in MITF expression, and showed lower expressions than normal group at 25, 50, and 100 μg/mL. Fermented T. radicans showed a significantly higher inhibitory effect of MITF protein expression than T. radicans. As a result, fermented T. radicans is expected to be used as a good material of whitening functional cosmetics.
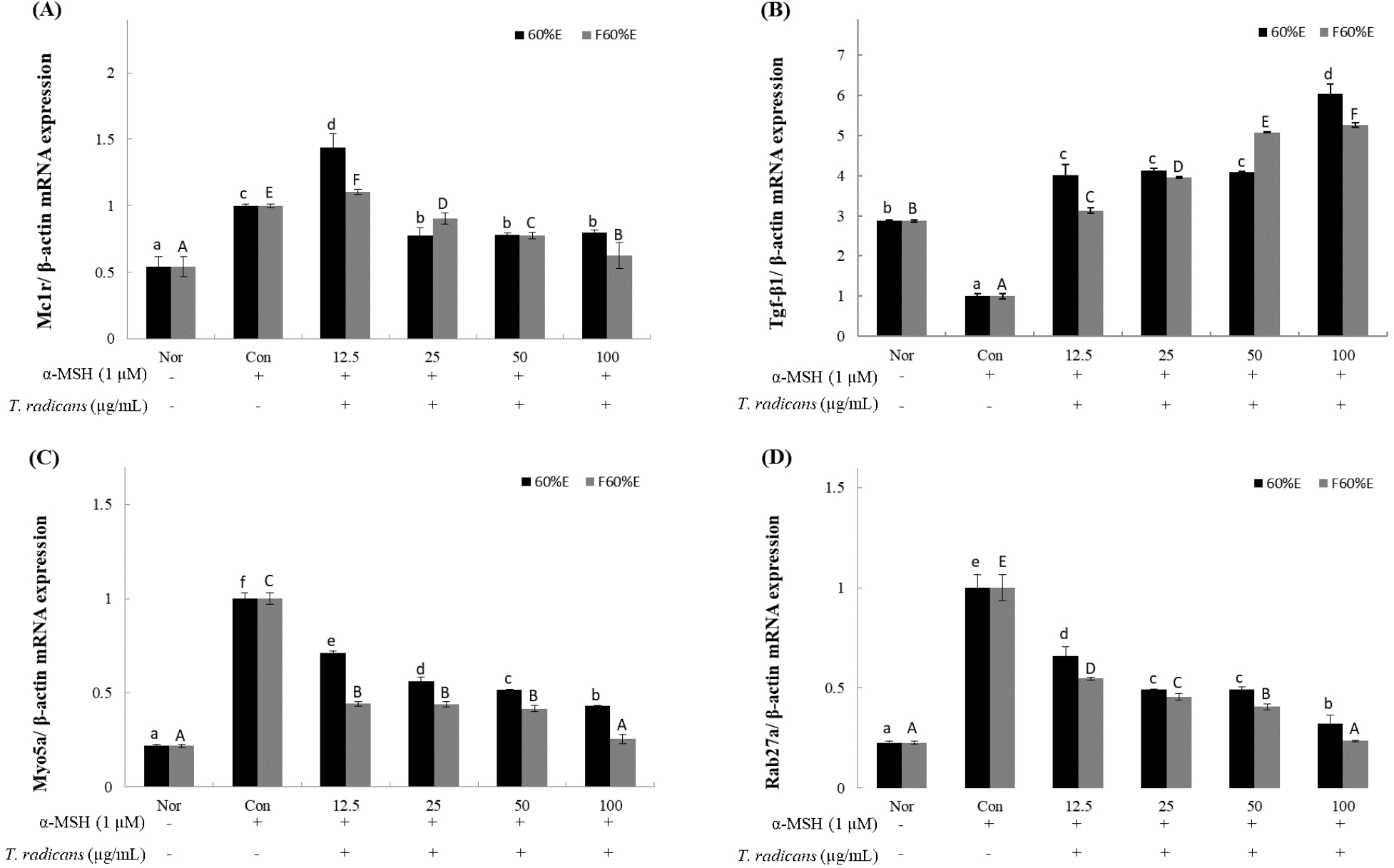
After confirming that T. radicans and fermented T. radicans inhibit the expression of MITF protein, to confirm the changes by RT-qPCR in gene expression of melanogenesis-related genes (MC1R, TGF-β1, Myo5a, Rab27a), freeze-dried samples of T. radicans and fermented T. radicans were treated at 12.5, 25, 50, and 100 μg/mL. MC1R, one of the key receptors involved in regulating the melanin synthesis, shown in Fig. 4A, T. radicans showed mRNA expression of 1.44, 0.77. 0.78, and 0.80, and fermented T. radicans showed expression of 1.10, 0.91, 0.78, and 0.63 at 12.5, 25, 50, and 100 μg/mL. Fermented T. radicans showed concentration-dependent decrease and showed effective inhibitory activities at 100 μg/mL. TGF-β1, activated by MC1R, is a potent inhibitor of melanin synthesis (Martínez-Esparza et al., 1997). As shown in Fig. 4B, T. radicans and fermented T. radicans both showed concentration-dependent increase in TGF-β1 expression, T. radicans showed mRNA expression of 4.02, 4.13, 4.10, and 6.05, and fermented T. radicans showed expression of 3.13, 3.96, 5.08, and 5.27 at 12.5, 25, 50, and 100 μg/mL. T. radicans and fermented T. radicans both showed effective activities compared to normal groups even at low concentrations, and results of TGF-β1 expression showed similar patterns with melanin contents. Myo5a and Rab27a, which regulates skin pigmentation during the melanosome transport process, showed in Fig. 4C and 4D. As shown in Fig. 4C, T. radicans showed Myo5a expression of 0.71, 0.56, 0.52, and 0.43, and fermented T. radicans showed expression of 0.44, 0.44, 0.42 and 0.25 at 12.5, 25, 50, and 100 μg/mL. As shown in Fig. 4D, T. radicans showed Rab27a expression of 0.66, 0.49, 0.49, and 0.32, and fermented T. radicans showed expression of 0.55, 0.45, 0.41, and 0.24 at 12.5, 25, 50, and 100 μg/mL. Results of Myo5a and Rab27a expression, T. radicans and fermented T. radicans both showed concentration-dependent decrease and showed similar patterns. Myo5a and Rab27a expressions of fermented T. radicans showed similar expression rates compared to normal groups at 100 μg/mL. As a result, fermented T. radicans showed higher inhibitory effects of expression of melanogenesis-related genes, confirming that bioconversion through fermentation inhibits the expression of melanogenesis-related gene. Fermented T. radicans expected to be used as a good material of whitening functional cosmetics.
4. Conclusions
In this study, Trigonotis radicans var. sericea was used to extract with 60% ethanol of T. radicans and T. radicans fermented with L. brevis and compare their whitening effects through bioconversion. The MTT assay to measure cell viability in B16-F10 cells, fermented T. radicans treated group showed higher cell viability than T. radicans, and confirmed significantly lower cell toxicity. And fermented T. radicans treated group showed lower melanin contents than T. radicans treated group at 25, 50, and 100 μg/mL. After confirming melanin production stimulated by α-MSH, expression of MITF was analyzed by western blot to confirm the effect on melanogenesis-related protein expression, and fermented T. radicans treated group showed lower expressions at 25, 50, and 100 μg/mL. RT-qPCR was performed to confirm the changes in gene expression of melanogenesis-related genes (MC1R, TGF-β1, Myo5a, Rab27a) by RT-qPCR. In MC1R expression, Fermented T. radicans treated group showed effective inhibitory activities at 100 μg/mL. In TGF-β1, T. radicans and fermented T. radicans treated groups showed effective activities compared to normal groups even at low concentrations. Results of Myo5a and Rab27a expression showed similar patterns, and Myo5a and Rab27a expressions of fermented T. radicans treated group showed effective inhibitory activities at 100 μg/mL. Western blot and RT-qPCR confirmed that fermented T. radicans has higher whitening effects through bioconversion. As a result, fermented T. radicans is expected to be a good material for whitening functional cosmetics.
