1. Introduction
Broccoli (Brassica oleracea var. italica) is a cruciferous vegetable with four petals forming a cross shape. It is rich in vitamins, minerals, and polyphenol compounds and contains unique bioactive substances called glucosinolates (Fenwick et al., 1983; Murillo and Mehta, 2001). Glucosinolates are secondary metabolites that exist only in cruciferous vegetables and are secreted by plants to defend themselves against external stimuli. They contain sulfur, are highly volatile, and have a unique spicy aroma (Lewis and Fenwick, 1987; Manchali et al., 2012). Glycosinolates have no biological activity; however, during cutting or chopping cruciferous vegetables for cooking or processing, they are hydrolyzed by an enzyme called myrosinase within the cruciferous vegetables. They are converted into hydrolysis products such as nitriles, epithionitriles, oxazolidine- 2-thiones, thiocyanates, and isothiocyanates, which exhibit various physiological activities (Dinkova-Kostova and Kostov, 2012; Wu et al., 2021).
Each cruciferous vegetable contains different and unique isothiocyanates, and they are known to have excellent antioxidant activity and excellent immune health effects (Rizwan and Masoodi, 2024; Sharma et al., 2016; Ting et al., 2020). In addition, it is reported to prevent cancer by increasing the activity of detoxification enzymes in the body, such as quinone reductase and glutathione-S-transferase (Fahey et al., 2001; Rizwan and Masoodi, 2024). In particular, glucoraphanin, a type of glucosinolate in broccoli, is hydrolyzed by myrosinase and converted to sulforaphane, which shows strong anticancer activity and has been reported to effectively prevent or reverse liver, hyperlipidemia, and cardiovascular diseases (Becker and Juvik, 2016; Conzatti et al., 2015).
Broccoli is prone to spoilage because of its high rates of respiration and transpiration, which cause significant economic losses and difficulties in postharvest storage, distribution, and supply management (Brennan and Shewfelt, 2010; Ilahy et al., 2020). Broccoli can be eaten fresh without being cooked, but it s also sometimes steamed or blanched in boiling water.
Further research is needed on various heat treatments to delay broccoli spoilage and preserve the quality of fresh products. In vegetables, components that are tightly bound to the tissue undergo physical and chemical changes during heat treatment, softening and loosening the cellular tissue, which can increase the elution and bioavailability of useful bioactive substances. In contrast, the heat-sensitive or water-soluble components are eluted and lost (Antony and Farid, 2022; Kim et al., 2014).
In this study, broccoli, which is the most widely known and consumed cruciferous vegetable, was analyzed for total polyphenols, total flavonoids, and major glucosinolate contents in the uncooked form and after boiling or steaming to determine antioxidant activity. The purpose of this measurement was to provide basic data for broccoli consumption.
2. Materials and methods
Broccoli grown in Jeju Island was purchased at a large supermarket in Seoul and used in the experiment. Standards for glucosinolate analysis, such as sinigrin, gluconapin, gluconasturtiin, glucoraphanin, and glucobrasscin, were purchased from Extrasynthese (Impasse Jacquard, Genay Cedex, France). Reagents for bioactive substance analysis, antioxidant activity measurements, and solvents for high- performance liquid chromatography (HPLC) and liquid chromatography-mass spectrometry analyses were purchased from Fisher Scientific (Fairlawn, NJ, USA), Junsei Chemical Co., Ltd (Tokyo, Japan), and Sigma-Aldrich Chemical Co. (St. Louis, MO, USA).
Broccoli was removed from the surface of foreign substances and washed thoroughly with running water to remove moisture, and the edible parts were cut into appropriate sizes, mixed well, and heat-treated using two methods (boiling and steaming). Fresh, unheated broccoli was used as a control. The boiling method was used to pour water equivalent to five times the weight of the sample (300 g) into a pot; when the water began to boil, the sample was placed in and boiled for 5 min, and then the sample was placed in a sieve to remove moisture. For steaming, water was poured into a steamer (Tefal, Seoul, Korea) and boiled. When the steam rose, 300 g of the sample was added and steamed for 5 min. Then, the sample was placed through a sieve to remove the moisture. After the heat treatment was completed, the sample was cooled, placed in an aluminum lunch box, and rapidly frozen in an ultra-low temperature freezer (DF-810, Ilshin Lab Co., Seoul, Korea) at -75°C. After the sample was completely frozen, it was dried in a freeze dryer (IlShin Biobase, Dongduchun, Korea), ground using a grinder (Hanil, Seoul, Korea), and passed through 30 mesh and 100 mesh sieves twice to obtain a powder. It was manufactured as a fine powder. The prepared sample was placed in a plastic tube, stored at -20°C, and used for subsequent analysis.
The total polyphenol content was analyzed using the Folin-Denis method (Vasco et al., 2008). First, 4 mL of distilled water was added to 1 g of the powder sample, sonicated at 40°C for 5 min, and centrifuged at 3,000 rpm for 10 min. Then, 0.5 mL of the supernatant was mixed with Folin’s reagent (0.5 mL) and allowed to react at room temperature for 3 min. Subsequently, 1.5 mL of 2% sodium carbonate (Na3CO3) was added to the reaction mixture, allowed to react in the dark for 2 h, and analyzed by measuring the absorbance at 760 nm using an enzyme-linked immunosorbent assay (ELISA) plate reader (Infinite M200 Pro, Tecan Group Ltd., San Jose, CA, USA). The total polyphenol content in the sample was expressed as μg gallic acid equivalents per gram of sample using a standard curve of gallic acid (6.25-100 μg/mL).
The total flavonoid content was analyzed according to the method described by Woisky and Salatino (1998). First, 4 mL of distilled water was added to 1 g of the powder sample, sonicated at 40°C for 5 min, and centrifuged at 13,000 ×g for 10 min. Then, 1 mL of a 2% aluminum chloride methanolic solution was added to 1 mL of the supernatant, and the mixture was allowed to react at room temperature for 15 min. The absorbance of the reacted solution was measured at 430 nm using an ELISA reader (Infinite M200 Pro, Tecan Group Ltd.), and the total flavonoid content of the sample was calculated as μg catechin equivalent per g of sample using the standard curve of catechin (6.25-100 μg/mL).
Glucosinolates in broccoli were analyzed using a method recognized by the International Organization for Standardization (ISO, 1992). First, 50 mg of freeze-dried broccoli powder was mixed with 70% boiling ethanol, reacted in a 70°C water bath for 5 min, and then centrifuged at 13,000 ×g for 20 min at 4°C. After loading the centrifuged supernatant onto an anion minicolumn filled with dextran gel (G-25 type) in a Bio-spin chromatography column (Bio-Rad Laboratories, Hercules, CA, USA), 75 μL of aryl sulfatase (EC 3.1.6.1, type H-1 from Helix pomatia) solution was loaded. Sulfur was separated from glucosinolates in the sample by incubation at room temperature for 12 h. While loading 3 mL of distilled water into the minicolumn three times, the glucosinolates from which sulfur was separated were collected separately and analyzed using HPLC (UltiMate 3000, Dionex, Sunnyvale, CA, USA). Desulfo-glucosinolates were analyzed at 227 nm using an inertsil ODS2 (C18) column (4.6×250 mm, GS Science, Tokyo, Japan), the column oven temperature was 35°C, and the mobile phase was flowing at 1.0 mL/min. The mobile phase was deionized water (solvent A) and 20% acetonitrile (solvent B), using a linear gradient of 1-99% solvent B for 18 min, 99% solvent B for 11 min, and 99-1% solvent B for 3 min. They were analyzed during the injection. Authentic glucosinolate standards were desulfated and used for peak identification and quantification. Concentrations of individual desulfo-glucosinolates were determined from the experimental peak area by analytical interpolation using an external standard calibration curve of each desulfo- glucosinolates across different ranges depending upon the glucosinolates and were expressed as μmol/g.
Freeze-dried broccoli powder (5 g) was extracted with 45 mL of distilled water or 80% ethanol in a constant-temperature water bath at 37°C for 2 h. After extraction, the aqueous or 80% ethanol fraction was concentrated using a rotary vacuum concentrator (EYELA, Rikakiki Co., Tokyo, Japan) to prepare a stock solution. The prepared stock solution was stored at -20°C and diluted to the desired concentration to measure the antioxidant activity.
The electron-donating ability of broccoli extract according to the heating and extraction method was measured using a 2,2-dipheny1-picrylhydrazyl (DPPH) assay (Cheung et al., 2003). First, 100 μL of extract of each concentration and 100 μL of 0.2 mM DPPH solution were added to a 96-well plate and reacted at 37°C for 30 min. The absorbance was measured at 515 nm using an ELISA reader, and the electron- donating ability of the sample to DPPH radicals was calculated by substituting the measured absorbance value into the following equation:
The 2,2′-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS) radical scavenging ability of broccoli extracts heated with different extraction solvents was measured using the method described by Re et al. (1999). First, 100 μL of the extract of each concentration and 100 μL of 0.2 mM ABTS solution were added to a 96-well plate and reacted at 37°C for 30 min. The absorbance was measured at 732 nm using an ELISA reader, and the electron-donating ability of the sample to the ABTS radical was calculated by substituting the measured absorbance value into the equation below:
The reducing power of the broccoli extract was analyzed using the method described by Oyaizu (1986). Phosphate buffer (200 mM, pH 6.6) and 1 mL of 1% potassium ferricyanide were sequentially added to 1 mL of broccoli extract according to the heating and extraction method and then reacted in a water bath at 50°C for 20 min. Next, 1 mL of 10% trichloroacetic acid solution was added, and the mixture was centrifuged at 13,500 ×g for 15 min. Distilled water (1 mL) and ferric chloride were added to 1 mL of the obtained supernatant and mixed, and the absorbance was measured at 720 nm.
All results are presented as the mean±SD. Statistical analyses were performed using the statistical analysis system Statistical Package for the Social Sciences (SPSS Inc., Chicago, IL, USA). For significant differences between each group, t-test or one-way analysis of variance was performed at the p<0.05 level, and significant differences were verified using Duncan’s multiple range test.
3. Results and discussion
Table 1 presents the total polyphenol and flavonoid contents of broccoli according to the cooking method and extraction solvent. The total polyphenol content was highest at 34.98 μg GAE/g of dry weight in the distilled water extract of fresh broccoli, and 21.66 μg GAE/g in the case of steamed broccoli, which was 38.08% lower than that in the fresh distilled water extract. In the 80% ethanol extract, the total polyphenol content was the highest at 37.08 μg GAE/g of weight in the broccoli that was not heat-treated, 28.73 μg GAE in steaming, and 28.04 μg GAE in boiling, which decreased by 22.52% and 24.38%, respectively, compared with the fresh sample. Regardless of heat treatment and cooking method, it was confirmed that the total polyphenol content was higher when extracted with 80% ethanol than with distilled water extract.
The total flavonoid content was measured using catechin as a standard. In the distilled water extract, the broccoli extract without heat treatment was the highest at 1.72 μg CE/g of weight, and the total flavonoid content was 1.49 μg CE and 1.39 μg CE in the steaming and boiling treatment groups, respectively, compared with fresh broccoli. They decreased by 13.37% and 19.19%, respectively. The total flavonoid content decreased by 12.97% and 16.76%, respectively in 80% ethanol extracts, compared with the fresh sample, to 1.85 μg CE/g of weight in broccoli without heat treatment, 1.61 μg CE in steaming, and 1.54 μg CE in boiling. Similar to the total polyphenol content, the total flavonoid content was also higher in samples extracted with 80% ethanol than with distilled water, regardless of cooking method. Cruciferous vegetables contain a variety of components with excellent physiological activity, and the degree to which these substances are destroyed varies depending on the cooking method. Additionally, since the solubility of these substances is different, the extent of extraction varies depending on the type and ratio of solvent, extraction time and temperature (Karanikolopoulou et al., 2021). Therefore, it is important to select an appropriate extraction solvent and heat treatment method. Water is the most widely used solvent in cooking and processing food and is effective in extracting active ingredients because of its high polarity (Pin et al., 2009). Additionally, ethanol is effective for eluting flavonoid components. Generally, when manufacturing plant extracts, an aqueous ethanol solution mixed with water and ethanol is advantageous for extracting phenolic compounds due to its high penetration ability (Wachtel-Galor et al., 2008). Polyphenol compounds decompose or oxidize when exposed to heat or sunlight for long periods (Chen et al., 2023; Koeppen and Roux, 1966).
The content of polyphenol substances in cruciferous vegetables changes depending on various cooking methods such as boiling, steaming, pressure cooking, and microwave ovens. Heat treatment intensity and time and the loss rate vary depending on the developed compound (Rangkadilok et al., 2002). Steaming was a better method to preserve phenolic substances. According the a previous study, boiling method reduced total glucosinolates by 64% and phenolic compounds by more than 70%. When turnip greens were boiled, about 64% of the flavonoids were reduced as water-soluble substances were eluted into the cooking water (Rangkadilok et al., 2002).
Steaming broccoli retains flavonoids and hydroxycinnamic acids without reducing them, whereas microwave heating or boiling for 3-15 min induces the loss of 30-90% of phenolic substances (Francisco et al., 2010). In particular, when food is boiled with a large amount of water, the surface area of the food increases as the food absorbs the water, and the cell walls are destroyed, causing phenolic substances that are tightly bound to the food cells to easily dissolve into the water (Francisco et al., 2010). According to a study by Wu et al. (2019), when broccoli and cauliflower are boiled in water, quercetin 3-O-sinafoyldiglucoside-7-O-glucoside, kaempferol-3-O-caffeoyldiglucoside-7-O-glucoside, Kaempferol-3-O-diglucoside-7-O-glucoside is produced. Boiling decreased the content of kaempferol-3-O-sinapoylsophoroside-7-O-glucoside, while steaming or heating using a microwave oven reduced the loss of these components compared to boiling (Wu et al., 2019). The reason why the loss of flavonoid components is greater after boiling compared to other recipes is thought to be because these components are decomposed by heat, and in particular, water-soluble flavonoid substances are dissolved in cooking water, reducing the amount remaining in cruciferous vegetables (Tiwari and Cummins, 2013). Therefore, steaming is considered a preferred cooking method than boiling in terms of lowering the rate of loss of polyphenol and flavonoid components and increasing the availability of soluble phenol and flavonoid components (dos Reis et al., 2015; Rennie and Wise, 2010).
The representative HPLC chromatogram of glucosinolate content according to the broccoli cooking method (fresh, steamed, and boiled) is shown in Fig. 1. Nine types of glucosinolates were identified in the broccoli extract: glucoiberin, progoitrin, glucoraphanin, sinigrin, glucoalyssin, gluconapin, glucobrasscin, gluconasturtin, and 4-methoxyglucobrasscin.
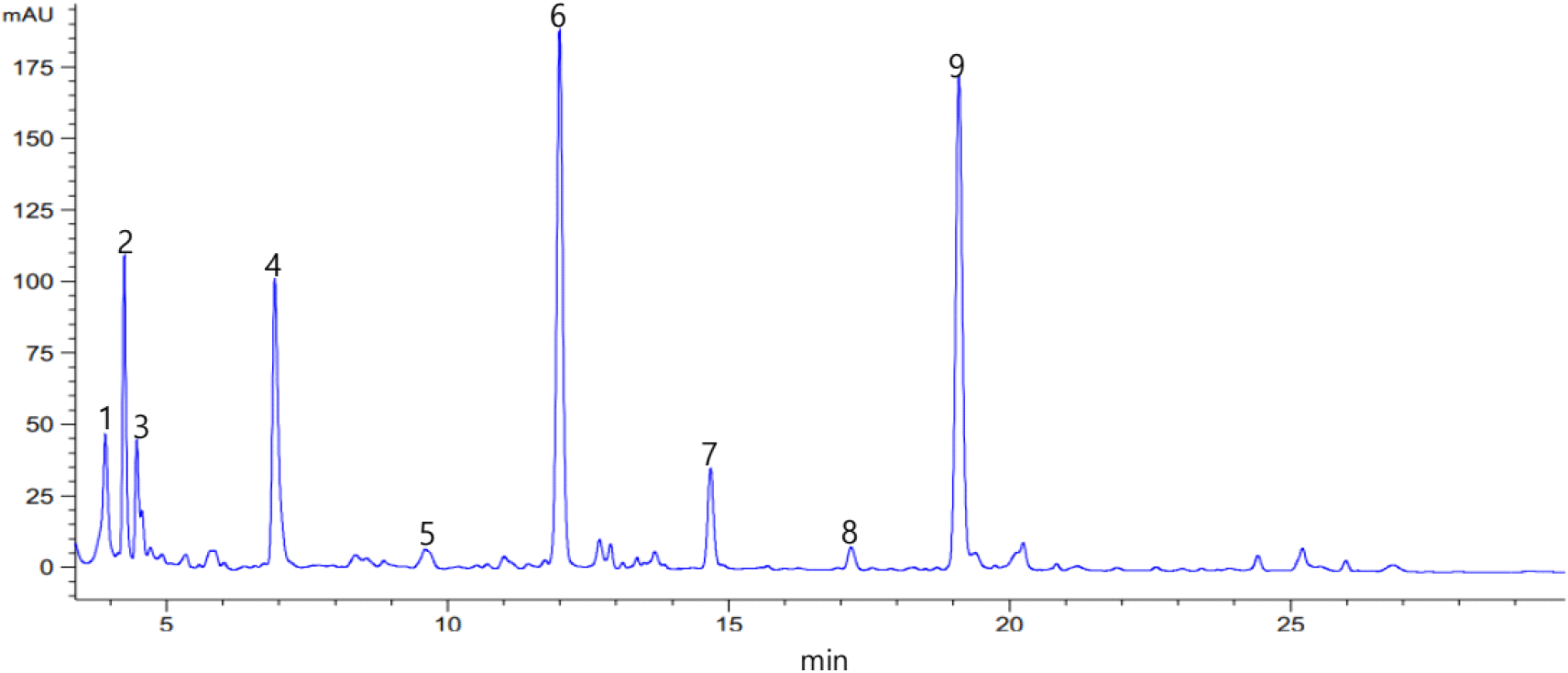
Table 2 shows the results of measuring and quantifying the glucosinolate content in broccoli using HPLC when the heat treatment method of broccoli was different. In fresh broccoli that was not heat-treated, a total of five species, including gluconapin, 4-methoxyglucobrasscin, progoitrin, sinigrin, and glucoraphanin, showed high contents of more than 100 pmol/g of dry weight. In fresh broccoli, glucoraphanin was detected in the highest amount at 416.72 pmol/g dry weight, followed by 4-methoxyglucobrasscin, progoitrin, and sinigrin at 366.98, 127.24, and 126.80 pmol/g, respectively. In case of steaming, gluconapin, 4-methoxyglucobrasscin, and progoitrin increased to 422.29, 386.49, and 147.25 pmol/g, by 1.34%, 5.32%, and 15.73%, respectively, compared with broccoli without heat treatment. Glucoiberin decreased in steamed broccoli compared to raw broccoli, but other glucosinolate contents increased. Briefly, glucoiberin content decreased by 2.31% compared with that in fresh broccoli. In the case of boiling with water, the contents of gluconapin, 4-methoxyglucobrasscin, and sinigrin were 405.44, 322.74, and 103.17 pmol/g, which decreased by 2.71%, 12.06%, and 18.64%, respectively, compared with broccoli that was not heat-treated. All other glucosinolate contents decreased by 5.74% to 18.64% compared with fresh broccoli, and the decrease was greater in the boiled than in steamed broccoli. These results confirmed that steaming increases the glucosinolate content compared with that in the fresh state without heat treatment, whereas boiling decreases the glucosinolate content. The total glucosinolates contained in cruciferous vegetables vary depending on the cooking method such as steaming, boiling, pressure cooking, and microwave, as well as the time and length of heat treatment. Additionally, different cooking methods affect the type and degree of loss of individual glucosinolates (Rangkadilok et al., 2002). Steaming is a method of better preserving total glucosinolates, and with the existing boiling method, total glucosinolates were reduced by 64%, and phenolic compounds were reduced by more than 70%. Aliphatic and indolic glucosinolates are similarly degraded (Czarniecka-Skubina, 2002; Howard et al., 1999; Zhang and Hamauzu, 2004).
The compounds were identified and quantified by comparing the chromatography with the authentic standards.
The cooking method is another factor that affects the breakdown of glucosinolates or sulforaphane in broccoli. Boiling causes significant losses through the thermal decomposition of glucosinolates (Baenas et al., 2019; Lafarga et al., 2018). The change in glucosinolate content due to steaming varies over time, depending on the cooking method (Lafarga et al., 2018; Zhang and Hamauzu, 2004). Cooking or heat treatment plays an important role in separating glucosinolates, the main bioactive substances of cruciferous vegetables, from cells and increases the extractability of these substances (Oliviero et al., 2012; Yuan et al., 2009). Compared to steaming or microwave cooking, boiling causes water-soluble glucosinolates to leach out into the cooking water, resulting in a significant loss of glucosinolates (Chiu et al., 2020). In Wang’s study (2021), the boiling reduced the glucosinolate content to a higher level compared to the steaming or microwave heating method of broccoli, which suggests that the boiling has a negative effect on the glucosinolate content (Gliszczynska-Swiglo et al., 2006). Therefore, it is important to use heating methods such as steaming or microwave and optimize cooking time to minimize the loss of glucosinolates and their decomposition products and to ensure that these substances can be efficiently used in the body.
Table 3 shows the DPPH radical scavenging activity of distilled water and 80% ethanol extract after freeze-dried broccoli obtained by different cooking methods. In broccoli water extract, DPPH radical scavenging activity increased depending on the concentration of the extract, with activity being higher in the order of raw > steamed > boiled broccoli. In addition, 400 μg/mL of fresh broccoli extract without heat treatment showed a DPPH radical scavenging activity of 42.00%, and steamed and boiled broccoli showed a DPPH radical scavenging activity of 31.62% and 28.77%, respectively. Moreover, 800 μg/mL of fresh broccoli extract without heat treatment showed a DPPH radical scavenging activity of 68.26%, and steamed and boiled broccoli showed a DPPH radical scavenging activity of 58.30% and 53.90%, respectively. In the 80% ethanol extract of broccoli, DPPH radical scavenging activity increased as the extract concentration increased, and at the same concentration, fresh broccoli showed higher DPPH radical scavenging activity than steamed and boiled broccoli. The 80% ethanol extract showed higher DPPH radical scavenging activity than the water extract. Additionally, 800 μg/mL of 80% ethanol extract from fresh broccoli showed 74.96% DPPH radical scavenging activity, while steamed and boiled broccoli showed 64.68% and 52.64% DPPH radical scavenging activity, respectively.
The ABTS radical scavenging activity according to the broccoli cooking method and extraction solvents is shown in Table 4. In broccoli water extract, ABTS radical scavenging activity increased as the concentration of the extract increased, and at the same concentration, ABTS radical scavenging activity was high in the order of fresh > steaming > boiling. Additionally, 400 μg/mL of fresh broccoli extract without heat treatment showed an ABTS radical scavenging activity of 33.47%, and steamed and boiled broccoli showed an ABTS radical scavenging activity of 26.05% and 22.26%, respectively. Moreover, 800 μg/mL of fresh broccoli extract without heat treatment showed an ABTS radical scavenging activity of 58.75%, while steamed and boiled broccoli showed an ABTS radical scavenging activity of 42.74% and 35.87%, respectively. In the 80% ethanol extract of broccoli, ABTS radical scavenging activity increased as the extract concentration increased. At the same concentration, fresh broccoli showed higher ABTS scavenging activity than steamed and boiled broccoli. The 80% ethanol extract showed higher ABTS radical scavenging activity than the water extract. In addition, 800 μg/mL of 80% ethanol extract from fresh broccoli showed an ABTS radical scavenging activity of 60.66%, and steamed and boiled broccoli showed an ABTS radical scavenging activity of 57.81% and 40.22%, respectively.
The reducing power of broccoli according to cooking and extraction solvents is shown in Table 5. In the aqueous extract of broccoli, the reducing power increased as the concentration of the extract increased; at the same concentration, the reducing power increased in the order of fresh > steamed > boiled. The absorbance of 400 μg/mL of fresh broccoli extract at 720 nm was 0.23, and the absorbance of steamed and boiled broccoli was 0.21 and 0.20, respectively. Additionally, 800 μg/mL of fresh broccoli extract without heat treatment showed an absorbance of 0.29, and steamed and boiled broccoli showed a reducing power of 0.27 and 0.26, respectively. The reducing power of the 80% ethanol extract of broccoli was higher than that of the aqueous extract. As the concentration of the extract increased, the reducing power also increased. At a concentration of 800 μg/g of 80% ethanol extract, the reducing power of uncooked broccoli extract was 0.34; for steamed broccoli, it was 0.32; and for boiled broccoli, it was 0.29. The reducing power decreased more when boiled with water than when heated with steam.
Antioxidant activity varies depending on the cooking method of broccoli, which includes the type and structure of antioxidants contained in broccoli, cutting method of vegetables, cooking method, cooking temperature, structure and synergistic effect of substances coexisting in vegetables, and bioavailability of antioxidants (Jiménez-Monreal et al., 2009). The present study confirmed that the antioxidant activity of unheated broccoli was the highest and that it decreased in broccoli heated by steaming and boiling. A study by Hameed et al. (2023) also confirmed that the antioxidant activity decreased when broccoli was steamed or boiled, which is thought to be due to the elution of heat-sensitive or water-soluble bioactive substances from broccoli. Multescu et al. (2019) measured the antioxidant power of four types of cabbage through DPPH scavenging activity by heating them in boiling water for 5-15 min. The DPPH radical scavenging activity decreased by 50% when the boiling time was increased to 15 min. Therefore, minimizing the amount of water and heating time when heating cruciferous vegetables, including broccoli, can be applied in real life by recognizing that minimizing the amount of water and heating time is a way to reduce the content of antioxidant substances and the rate of loss of glucosinolates.
4. Conclusions
Broccoli was prepared by boiling or steaming in water, then freeze-dried to make powder, extracted with distilled water or 80% ethanol, and measured for bioactive substances and antioxidant activity contained in the extract. Broccoli that was not heated was compared as a control.
Total polyphenols were 34.98 μg GAE/g in the distilled water extract of broccoli that was not heat-treated, 21.66 μg GAE/g in the steamed case, and 21.56 μg GAE/g in the boiled case, which decreased compared to the fresh sample, but the difference between steaming and boiling treatments didn't show up. Flavonoids were highest at 1.85 μg CE/g in 80% ethanol extract in broccoli that had not been heat- treated, 1.61 μg CE/g in steaming, and 1.54 μg CE/g in boiling, which decreased the total flavonoid content by 12.97% and 16.76%, respectively, compared to the fresh sample. Regardless of the heating mehod of broccoli, it was found that when broccoli was extracted with 80% ethanol, it contained more total polyphenols and total flavonoids than when extracted with distilled water. Fresh broccoli contains a total of nine types of glucosinolates: glucoiberin, sinigrin, glucoalyssin, progoitrin, glucoraphanin, gluconapin, glucobrasscin, 4- methoxyglucobrasscin, and gluconasturtin. Although there were some differences depending on the type of glucosinolates, compared to fresh broccoli, the glucosinolate content increased in steamed broccoli, while the glucosinolate content decreased by 5.74-18.64% in boiled broccoli. The antioxidant activity of broccoli extract measured by reducing power, ABTS, and DPPH radical scavenging activity increased as the concentration of broccoli extract increased, and the distilled water extract showed higher antioxidant activity than the 80% ethanol extract. Antioxidant activity decreased in samples using steaming or boiling methods compared to fresh samples, and the decrease in antioxidant activity in boiling samples was greater than in steaming samples. Therefore, in order to consume the bioactive substances contained in cruciferous vegetables, including broccoli, without loss, it is considered preferable to steam them or consume them fresh rather than boiling them in water.
