1. Introduction
Plants are a major source of first-line medicine for local communities around the world (Mbosso et al., 2015), and two-thirds of the world’s population uses traditional therapies due to their efficacy and the high cost of pharmaceutical products (Tagboto and Townson, 2001). Approximately 6% of the world’s plants have been screened for their bioactivity, and only 15% of these have been evaluated for phytochemicals such as phenolic compounds, alkaloids, and terpenoids, which are used to develop antioxidant, anticancer, antimicrobial, and anti-inflammatory pharmaceuticals (Verpoorte, 2000). Many antimicrobial and anticancer medicines now on the market contain plant-derived natural material. Research is continuing into the wide range of traditionally therapeutic plants not yet investigated.
Pawpaw (Asimina triloba [L.] Dunal) is a commercially important member of the Annonaceae family that includes custard apple, cherimoya, sweetsop, and soursop (Brannan et al., 2015). It was historically harvested in the wild but is now cultivated as an orchard crop in several regions of America, Italy, Portugal, Belgium, China, Japan, and Korea (Brannan et al., 2015; Nam et al., 2017). It can be grown without pesticides (Ferreira et al., 2011) and is tolerant of a range of climates (Callaway, 1993). However, pawpaw is not well known to people, and although it has been steadily cultivated by farmers since 2015, it is difficult to commercialize it.
Since pawpaw is associated with a number of health benefits, various studies have been investigated its antioxidant, anticancer, and trematocidal activities (Brannan and Salabak, 2009; Farag, 2009; Kobayashi et al., 2008; McLaughlin, 2008; Nam et al., 2018; Nam et al., 2021; Ratnayake et al., 1993). In particular, acetogenin contained in the pawpaw is known to have strong anticancer activity, and a medicine made from pawpaw extract containing 50 types of acetogenin is also on sale. However, domestic research on the anticancer activity of pawpaw is minimal and limited to specific cancer cells. In addition, there have been few studies on antibacterial effects according to various pawpaw parts. Therefore, we aimed to investigate effective antibacterial and anticancer activities of materials derived from pawpaw as a promising source of phytochemicals.
2. Materials and methods
Pawpaw trees (n=150; 2-3 years old, 1-2 m height) were obtained from a farm in Okchon, Korea (average annual temperature of 13.0°C, average annual relative humidity of 66.7%, average annual wind velocity of 1.9 m/s; total annual rainfall 1,458.7 mm). A voucher specimen was authenticated by Dr. Otto Jahn (United States Department of Agriculture/Agricultural Research Service) and was deposited in the herbarium by the U.S. National Plant Germplasm System. After cleaning the trees, the leaves, twigs, and roots were separated and immediately washed with cold water to remove any soil, dust, or insects. The samples were then lyophilized using a pilot plant freeze dryer (LP100, Ilshin Lab Co., Daejeon, Korea), pulverized with a grinder (Blixer, Robot Coupe USA, Jackson, MS, USA), and passed through a 40-mesh sieve to remove large debris. Samples were stored in a deep freezer at −70°C until use.
Mueller Hinton Agar (MHA) and kanamycin were purchased from Difco (Sparks, MD, USA). Roswell Park Memorial Institute (RPMI) and Dulbeco’s Modified Eagle’s Medium (DMEM) were obtained from Gibco (Grand Island, NY, USA), and fetal bovine serum (FBS) and streptomycin-penicillin were purchased from Welgene (Seoul, Korea). Tetrazolium-based 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) solution was supplied from Sigma-Aldrich (St. Louis, MO, USA). All solvents and chemicals used were of analytical grade.
Water and methanol, which are most commonly used when measuring various biological activities, show different activities depending on the properties of the functional compounds. Briefly, water can extract aqueous compounds, and water-methanol mixed solvent can extract both aqueous and nonaqueous compounds. Accordingly, pawpaw leaf, twig, and root were extracted in distilled water or 80% methanol in at least triplicate to obtain pawpaw leaf, twig, and root distilled water extracts (PLW, PTW, and PRW, respectively) and methanolic extracts (PLM, PTM, and PRM, respectively). Briefly, distilled water or 80% methanol was added to each sample at a ratio of 1:10 (w/v) with continuous agitation on a horizontal shaker (BS-21, Jeio Tech, Daejeon, Korea) for 24 h at 25°C. The extracts were centrifuged at 11,325 ×g for 20 min at 4°C and the supernatant was recovered using vacuum filtration. Sample extracts were concentrated using a rotary evaporator (R-210, Buchi, Flawil, Switzerland) and lyophilized. The dried extracts were weighed to calculate the yield and stored at −70°C until further analysis.
The test strains for the agar diffusion assay were obtained from the Korean Collection for Type Cultures (KCTC) at the Korea Research Institute of Bioscience and Biotechnology (Daejeon, Korea) and Korean Culture Center of Microorganisms (KCCM) (Seoul, Korea), and included gram-positive bacteria (Bacillus cereus KCCM 40935, Bacillus subtilis KCTC 3135, Clostridium perfringens KCTC 3269, Corynebacterium xerosis KCTC 3435, Listeria monocytogenes KCTC 13064, methicillin-resistant S. aureus KCCM 40510, Propionibacterium acnes KCTC 3314, Staphylococcus aureus KCCM 12103, Staphylococcus epidermidis KCCM 40416, and Streptococcus mutans KCCM 40105) and gram-negative bacteria (Campylobacter jejuni KCTC 5327, Cronobacter sakazakii KCTC 2949, Escherichia coli KCTC 2441, E. coli O157:H7 KCCM 11862, Proteus vulgaris KCTC 2512, Pseudomonas fluorescens KCTC 12453, Salmonella typhimurium KCTC 2514, Vibrio fluvialis KCTC 2473, Vibrio parahaemolyticus KCCM 11965, and Yersinia enterocolitica KCCM 41657) (Table 1). After passaging twice in tryptic soy broth medium (Difco), the strains were activated in MH broth (MHB) at 37°C for 24 h. Bacterial suspensions of each strain were prepared in sterile water and stored at 4°C for 24 h.
The antimicrobial activity of A. triloba extracts was evaluated using a modified version of the Kirby-Bauer disc diffusion method (Bauer et al., 1966; Rios et al., 1988). The extract concentrations were adjusted with water or 80% methanol to 100, 250, 500, 750, and 1,000 mg/mL and sterilized by passage through a 0.45 μM syringe filter. A 50 μL volume of these extracts was injected onto a 6 mm sterile disc (Advantec Toyo Roshi Kaisha, Tokyo, Japan) and the solvent was evaporated under an aseptic hood, yielding discs containing 5, 12.5, 25, 37.5, and 50 mg crude extract. A standard kanamycin disc (30 μg/disc) served as positive control and distilled water or 80% methanol served as negative controls for the antimicrobial test.
A double layer of medium was used to determine the inhibition zone of bacteria. The bottom and top layers of MHA contained 1.5% and 0.75% agar, respectively. The turbidity of the bacterial suspension was adjusted to 0.5 McFarland standard. The bottom layer was spread in the dish and the molten top agar was mixed with 100 μL bacterial suspension and immediately poured on the bottom layer. Sample filter paper discs were placed on the dish followed by incubation at 37°C for 20 h. Antimicrobial activity was determined by measuring the diameter of the clear zone (mm) for triplicate samples.
MIC, defined as the lowest concentration of antimicrobial substance that can inhibit microbial growth (Edziri et al., 2012; Sharma et al., 2012), was determined using broth microdilution for 20 pathogens using 96-well micro test plates (SPL Life Sciences, Seoul, Korea). Briefly, a bacterial suspension was diluted to 1.5×108 cfu/mL using MHB and 100 μL was seeded in a 96-well plate, followed by treatment with an equal amount of sample extract (0.006 mg/mL to 100 mg/mL). The plate was then incubated at 37°C for 20 h. The optical density of the culture was measured at 600 nm using VersaMax microplate reader (Molecular Devices, Sunnyvale, CA, USA). MBC was investigated for each well showing growth inhibition. Bacterial cells from the MIC test plate were sub-cultured on an agar plate by pouring them onto the agar surface and incubating at 37°C for 20 h. The concentration at which no bacterial growth occurred on the plate was considered the MBC (Akinyemi et al., 2005).
RAW 264.7 cell lines and human cancer cell lines from the Korean Cell Line Bank (KCLB; Seoul, Korea) were used to assess the anticancer effects of pawpaw extracts: RAW 264.7 (KCLB No. 40071), HT-1080 fibrosarcoma (KCLB No. 10121), HeLa cervical cancer (KCLB No. 10002), HepG2 hepatocellular carcinoma (KCLB No. 88065), and AGS stomach cancer (KCLB No. 21739). Cells were cultured in RPMI or DMEM containing 10% FBS and 100 unit/mL streptomycin-penicillin at 37°C and 5% CO2.
RAW 264.7 or 4 human cancer cell lines (2-4×104 cells/well) were treated with various concentrations (0, 2.5, 10, 50, 100, 200 μg/mL) of pawpaw extract in 96-well micro test plates. The treated plates were incubated for 24 h or 48 h at 37°C in a 5% CO2 incubator (BB 15, Thermo Fisher Scientific, Langenselbold, Germany). Following this, 20 μL MTT solution (5 mg/mL) was added to the wells and allowed to react for 2 h. The medium was removed by suctioning and 100 μL dimethyl sulfoxide was added to each well. Optical density at 540 nm was measured on a microplate reader (Infinite M200, Tecan, Männedorf, Switzerland) (Nam et al., 2018). The cytotoxicity of pawpaw extracts was expressed as the inhibition concentration (IC) of the pawpaw extracts that yielded 50% growth inhibition of human cancer cells.
3. Results and discussion
Antibacterial activities of six pawpaw extracts (PLW, PTW, PRW, PLM, PTM, and PRM) were evaluated using agar diffusion assay at concentrations of 5, 12.5, 25, 37.5, and 50 mg/disc in 20 strains of bacteria, including pathogenic microorganisms.
The antibacterial activity of each part of the pawpaw was very diverse (Tables 2 and 3). PLW showed inhibitory activity against B. cereus, C. perfringens, C. xerosis, and S. aureus among gram-positive bacteria (6.33-17.33 mm clear zone), and exhibited inhibitory activity against C. sakazakii, E. coli, E. coli O157:H7, P. fluorescens, P. vulgaris, S. typhimurium, V. fluvialis, V. parahaemolyticus, and Y. enterocolitica among gram-negative bacteria (6.00-15.33 mm clear zone). PTW showed inhibitory activity against B. cereus, B. subtilis, C. perfringens, Methicillin-resistant S. aureus, and S. aureus among gram-positive bacteria (6.33-19.00 mm clear zone), and exhibited inhibitory activity against E. coli, E. coli O157:H7, P. fluorescens, S. typhimurium, V. fluvialis, V. parahaemolyticus, and Y. enterocolitica among gram-negative bacteria (6.00-15.67 mm clear zone). PRW no exhibited inhibitory effect on gram-positive bacteria except for B. cereus, B. subtilis, and C. perfringens, and showed broad inhibitory activity against some gram-negative bacteria (6.00-20.50 mm clear zone). PLM showed inhibitory effect on gram-positive bacteria including B. cereus, C. perfringens, Methicillin-resistant S. aureus, S. aureus, and S. epidermidis (6.33-20.80 mm clear zone) and exhibited effect on gram-negative bacteria including V. fluvialis and Y. enterocolitica (6.00-12.33 mm clear zone). PTM showed inhibitory activity against all gram-positive bacteria except for P. acnes and S. mutans, and exhibited inhibitory effect on gram-negative bacteria including C. jejuni, P. vulgaris, V. fluvialis, V. parahaemolyticus, and Y. enterocolitica, ranged from 6.00-25.67 mm clear zone). PRM, at 50 mg/disc, exhibited potent, broad-spectrum antibacterial activity against all tested gram-positive and -negative strains, producing inhibition zones ranging from 9.67-29.50 mm. It was also the only sample that inhibited the growth of P. acnes and S. mutans. P. acnes is a pathogen that prefers anaerobic conditions and is related to dermal acne (Kirschbaum and Kligman, 1963). S. mutans is another anaerobic pathogen which contributes to dental caries (Persson et al., 1998). Consequently, pawpaw is under study for improving the quality of toothpaste. Based on these observations, PRM has potential applications as an active ingredient in cosmetics or toothpaste. C. perfringens was inhibited intensely by all pawpaw extracts. In particular, PTM showed stronger inhibitory activity than that of other extracts, presenting a clear zone of 30.33 mm at 50 mg/disc. C. perfringens is a spore-forming pathogen and is one of the most common causes of food poisoning in the United States (Kenneth, 1994). As such, PTM extract may be applicable in food preservatives.
2) PLW, pawpaw leaf water extract; PTW, pawpaw twig water extract; PRW, pawpaw root water extract; PLM, pawpaw leaf methanolic extract; PTM, pawpaw twig methanolic extract; PRM, pawpaw root methanolic extract.
Gram-positive bacteria were more inhibited by pawpaw methanol extracts than water extracts, and gram-negative bacteria were more inhibited by water extracts than methanol extracts. For example, PLW, PTW, and PRW had no antibacterial activity against 4 gram-positive bacteria (L. monocytogenes, P. acnes, S. epidermidis, and S. mutans) that were inhibited by PLM, PTM, or PRM. However, they had antibacterial activity against 4 gram-negative bacteria (E. coli, E. coli O157:H7, P. fluorescens, and S. typhimurium) that were not inhibited by PLM and PTM. According to a previous study, 4 extracts (n-hexane, methanol, ethanol, and distilled water) from guava contain various phytochemicals including phenols, tannins, saponins, terpenoids, flavonoids, and glycosides. Among guava extracts, a distilled water extract was the only one containing saponins (Biswas et al., 2013). In addition, saponin content of dandelion stem was higher in water extract than in methanol extract (Mir et al., 2013). Saponin fractions obtained from Gymnema sylvestre and Eclipta prostrata were more efficacious against gram-negative bacteria than gram-positive bacteria (Gopiesh Khanna and Kannabiran, 2008). Therefore, we suggest that the greater efficacy of pawpaw water extract against gram-negative bacteria may be due to saponins contained in the water extract.
The greater antibacterial effects in methanol extracts is consistent with previous reports from sweetsop (Annona squamosa), a co-familial species with pawpaw (Patel and Kumar, 2008), possibly because bioactive compounds have greater solubility in alcohol than in water. Our previous study indicated that PLM, PTM, and PRM had higher phenolic and flavonoid contents than PLW, PTW, or PRW (Nam et al., 2017). In addition, other studies have shown that high phenolic and flavonoid contents are associated with greater antibacterial effect (Duman et al., 2009; Pascoal et al., 2014); as such, the antimicrobial activities of pawpaw may be related to these compounds.
To assess the antimicrobial activity of the six pawpaw extracts, we measured the MIC and MBC values (Table 4). MIC and MBC of PLW against gram positive bacteria were 12.5-50 mg/mL, and 25 mg/mL, respectively, and MIC and MBC against gram negative bacteria were 3.13-50 mg/mL, and 12.5-100 mg/mL, respectively. PTW showed MIC values of ranged from 25-100 mg/mL against gram positive bacteria, and ranged from 12.5-100 mg/mL against gram negative bacteria. MIC and MBC of PRW against gram positive bacteria were ranged from 6.25-100 mg/mL, and 12.5-100 mg/mL, respectively, and MIC and MBC against gram negative bacteria were ranged 6.25-100 mg/mL, and 6.25-100 mg/mL, respectively. PLM exhibited MIC values of ranged from 3.13-100 mg/mL against gram positive bacteria, and ranged from 12.5-50 mg/mL against gram negative bacteria. MIC and MBC of PTM against gram positive bacteria were ranged from 0.78-100 mg/mL, and 3.13-100 mg/mL, respectively, and MIC and MBC against gram negative bacteria were ranged 3.13-50 mg/mL, and 25-100 mg/mL, respectively. MIC and MBC of PRM against gram positive bacteria were ranged from 0.78-25 mg/mL, and 3.13-100 mg/mL, respectively, and MIC and MBC against gram negative bacteria were ranged 3.13-25 mg/mL, and 6.25-100 mg/mL, respectively. Briefly, it was confirmed that the MIC and MBC values of PRM were the lowest for most bacteria.
In particular, the highest efficacy against S. mutans was observed in PRM, which had MIC of 0.78 mg/mL. PTM had the second-highest antibacterial activity, and showed inhibition against 15 strains, though not against P. acnes, E. coli, E. coli O157:H7, S. typhimurium, or E. sakazakii. The highest antibacterial activity was observed against C. xerosis and C. perfringens, with MIC values of 0.78 mg/mL, respectively. PLM inhibited the growth of all bacterial strains except for S. typhimurium, P. fluorescens, and V. parahaemolyticus. PLW had a relatively strong antibacterial effect compared with other water extracts, especially against Y. enterocolitica, with an MIC value of 3.13 mg/mL. PTW and PRW showed the lowest antibacterial activities against most of strains. As indicated in the diffusion test, bacterial growth was more effectively inhibited by methanol than by water extracts. Briefly, the most potent antibacterial activity was attributable to PRM, with MBC values against B. subtilis, C. xerosis, and C. perfringens of 3.13 mg/mL, respectively, which is lower than other samples. The MBC value of PTM against C. perfringens was also 3.13 mg/mL, indicating strong inhibition of bacterial growth.
The MBC/MIC ratio is termed the MIC index, and can be used evaluate whether an extract has a bactericidal (MIC index≤4) or bacteriostatic (4<MIC index<32) effect (Benamrouche et al., 2016; Hellal et al., 2017). The MIC index of PRM showed the strongest antibacterial activities, ranging from 2.0 to 32.1 for different bacteria strains. Its MIC index was <4 in 13 strains. PRM exhibited a bacteriostatic effect on B. cereus and methicillin-resistant S. aureus with an MIC index of 16.0, respectively, indicating that although it may inhibit their growth, it is not capable of killing them. Meanwhile, PTM showed bactericidal effects on B. cereus, B. subtilis, methicillin-resistant S. aureus, S. epidermidis, C. perfringens, C. jejuni, V. fluvialis, and P. vulgaris with MIC indices ranging from 1.0 to 4.0. However, it showed no bactericidal or bacteriostatic effects against C. xerosis, despite its low MIC value of 0.78 mg/mL. As such, PTM can be considered as having mild action against C. xerosis.
Patel and Kumar (2008) reported that the MIC values of petroleum ether, chloroform, methanol, and water extracts from A. squamosa against S. aureus were above 1,100, 1,100, 530, and 1,100 μg/mL, respectively. In addition, Edziri et al. (2012) showed that the MIC and MBC values of methanol and water extracts from Tunisian vegetables against E. coli, P. aeruginosa, S. aureus, and E. faecalis ranged from 0.312 to 10 mg/mL. The MIC values of Trilepisium madagascariense stem bark extracts obtained in various solvents against gram-positive and -negative bacteria ranged from 0.78 to 25 mg/mL (Teke et al., 2011). Annona reticulata had high antimicrobial activities against B. subtilis, E. faecalis, S. aureus, and E. coli with MIC and MBC values of 1-3 mg/mL, but its antimicrobial activities were different from one another or had no inhibitory effect (Sangeetha et al., 2016). Accordingly, we suggest that the antimicrobial activities in plants are affected by plant species, bacterial strain, and extracting solvents.
Cytotoxicity of the six pawpaw extracts was evaluated in RAW 264.7 cell lines and 4 human cancer cell lines (HT-1080, HeLa, HepG2, and AGS) using the MTT assay. All pawpaw extracts showed more than 80% cell viability against the RAW 264.7 cell lines (data not shown). All the pawpaw extracts showed inhibitory activity against cancer cells in a concentration-dependent manner (Fig. 1). There was no effect below 10 μg/mL concentration, but an inhibitory effect was observed at higher concentrations. In particular, in the case of HT-1080, PTM at a concentration of 50 μg/mL showed a viability of 65.52%, and at a concentration of 100 μg/mL, a viability of 46.79%. At a concentration of 200 μg/mL, an inhibitory effect of more than 80% was observed. HeLa, HepG2, and AGS were also confirmed to be the most active with PTM at a concentration of 200 μg/mL showing viability of 19.95%, 30.06%, and 10.94%, respectively. These results indicated that PTM has the highest inhibitory effect against 4 human cancer cell lines.
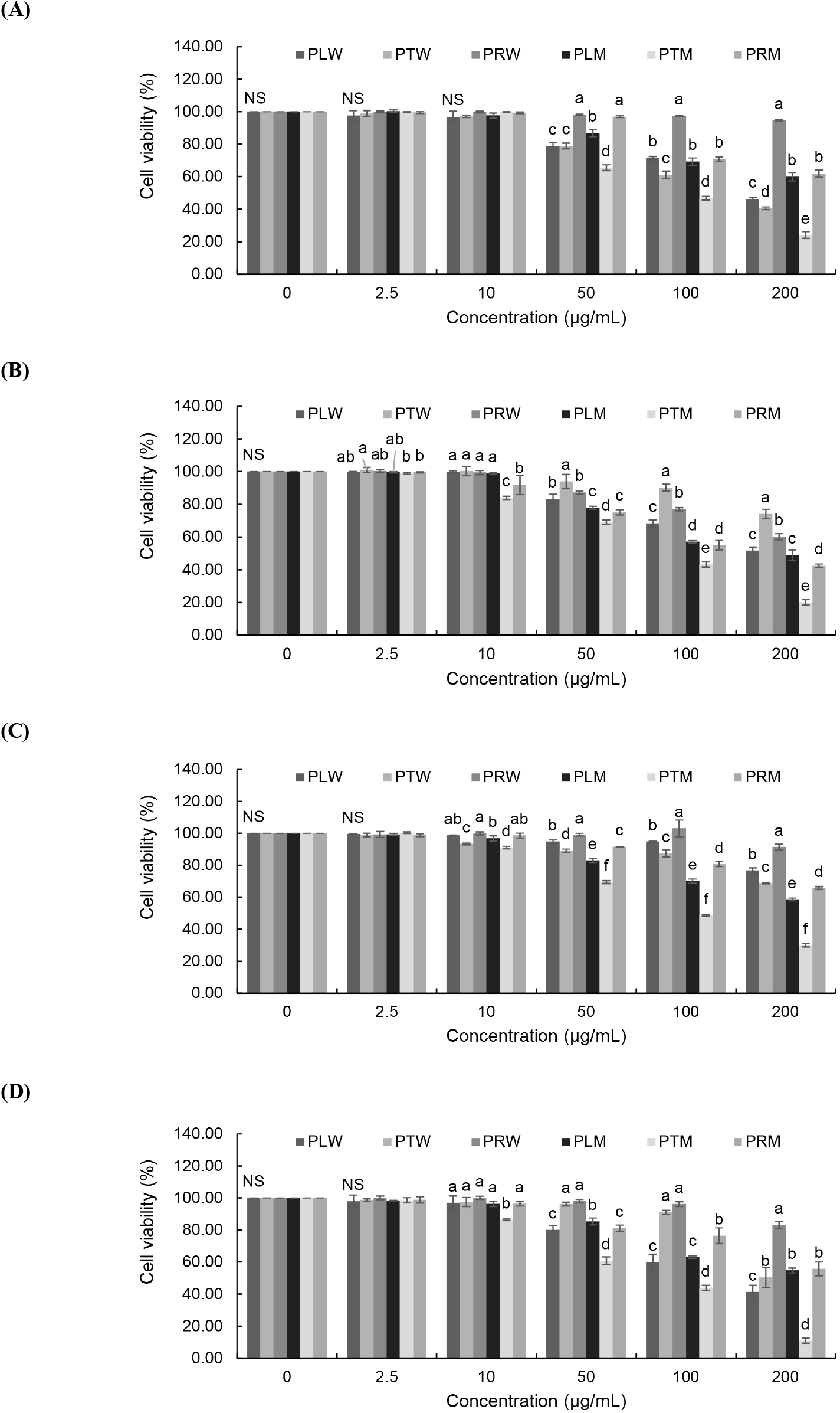
The IC50 values for anticancer activity are shown in Table 5. PTM had the strongest inhibitory effect against all cell lines except for HeLa cells, with IC50 values ranging from 64.57 to 128.60 μg/mL. The anticancer activity of PTM against fibrosarcoma HT-1080 was the highest, at twice that of the compound with the second-highest activity. In addition, five samples (PLW, PTW, PRW, PLM, and PRM) showed only 50% growth inhibition of HepG2 cells at a concentration of 200 μg/mL, while PTM inhibited this line at an IC50 value of 68.99 μg/mL. AGS stomach cancer cells were significantly inhibited by PLW and PTM, with respective IC50 values of 135.68 and 70.48 μg/mL. Cervical cancer HeLa cells were inhibited by methanol extracts, while the anti-proliferative activity of water extracts had negligible IC50 values of more than 200 μg/mL. As such, we can regard the cytotoxicity of PTM against HT-1080, HepG2, and AGS cell lines as the highest among the extracts.
The extract of A. squamosa pulp showed a weak influence on the HepG2 hepatocellular carcinoma cell line, with an IC50 value of 99 μg/mL (El-Darier and Abdelhady, 2017). Hashemi et al. (2017) demonstrated that ethanol extract of black tea inhibited the proliferation of AGS gastric cancer cells with an IC50 value of 264.3 μg/mL. Compared to these results, PTM had potent anti-proliferative activity. In addition, previous studies investigating the effects of pawpaw extracts have reported that the twig extract had higher acetogenin content and stronger pesticidal effect than extracts derived from other parts of the plant (McLaughlin, 2008; Ratnayake et al., 1993), and anticancer activity has been widely attributed to the Annonaceous acetogenin (McLaughlin, 2008; Nam et al., 2018). Accordingly, we suggest that the anticancer activity of PTM is related to acetogenin.
4. Conclusions
PRM was most effective in inhibiting the growth of all tested bacteria, and PTM was the most potent inhibitor of HT-1080 fibrosarcoma, HepG2 hepatocellular carcinoma, and AGS stomach cancer cell lines. This study is the first to demonstrate the antibacterial and anticancer activities of different parts of Korean pawpaw extracts. Although additional studies are needed to identify the precise compounds responsible for these effects, our findings indicate that Korean pawpaw extracts have potential medicinal value for the treatment of infections and cancer.











