1. Introduction
A poultry egg is a naturally preserved biological food item that is composed of a shell with four layers: shell membrane, mammillary knobs, palisade layer, and cuticle. The shell is a porous structure, and the pores are sealed partially with the proteinaceous cuticle, which allows permeability to gases while restricting the entry of micro-organisms to egg content (Belitz et al., 2009; Hunton, 2005). The shell membrane contains enzymes such as lysozyme and N-acetylglucosaminidase, which are considered bacteriolytic enzymes (Guha et al., 2019) that help ensure the natural protection of the egg. However, rapid deterioration of egg quality occurs during storage. The Freshness of an egg is the key characteristic that relates to its quality. Some studies discuss the possibility of bacterial entry soon after laying due to the presence of an immature cuticle for a short period (Miyamoto et al., 1998). In addition, changes in pH and water content of the yolk, changes in protein conformation, stretching and weakening of the vitelline membrane, and egg white thinning occur during the storage (Huang et al., 2012). Egg quality is mainly related to consumers’ acceptance. Other than the direct consumers of eggs, egg product manufacturers, such as producers who engage in producing albumen products, focus highly on the interior quality of eggs because
higher quality eggs assist in better component separation. Several parameters such as egg weight, freshness, cleanliness, Haugh unit (HU), yolk, and albumen indexes are used to measure the quality (Duman et al., 2016; Jones and Musgrove, 2005). These quality parameters are mainly divided into two general groups, such as internal (aspects related to air cell, yolk, and albumen) and external (shell shape, texture, and cleanliness) quality factors, which are used in the market to categorize the eggs into three grades as AA, A, and B. AA is considered the highest egg quality which decreases to A, and then to B with the time. The eggs below the B quality are inedible or loss (USDA, 2024a).
Other than grading, the shelf life of eggs in the market poses a critical concern. Storage temperature and humidity are two major factors that decide the shelf life of an egg. Based on records, eggs can be stored at 7°C (60% relative humidity) for 20 days, are not fit to consume after two weeks of storage at 37°C and should not be stored for more than one week where the ambient temperature rises to a level of 40°C (Kumari et al., 2020). According to the literature, storing eggs at room temperature was practiced in countries like India and Thailand at the market level, while further revealing the fact that 10% of consumers in Korea and 16% of consumers in Thailand and India had stored raw eggs at room temperature (Koppel et al., 2014). In addition, eggs are frequently stored at ambient temperature in retail markets of developing countries (Omana et al., 2011). Labeling the grade of eggs is common in markets to convey the quality. Size, weight, and grade description are considered important facts to be on the label for buyers (FAO, 2024). Therefore, pragmatic identification of variations that occur in quality parameters during storage of eggs at room temperature benefits direct consumers while purchasing and consuming. Apart from direct consumers, producers are also benefited since they are responsible for providing a safe and quality product to the market. Hence, the current study was designed to investigate the impact of the room temperature on the quality parameters of shell eggs under the ambient temperature (25°C±2) while discussing the deterioration of the grade during its shelf life.
2. Materials and methods
A total of 150 newly laid eggs were collected from a commercial farm where 28-week-old Hyline brown hens were reared and fed under a battery cage system with a ration containing 17.60% (w/w) crude protein, 7.23% (w/w) fat, 4.18% (w/w) crude fiber, and 14.62% (w/w) ash. Eggs were transported to the General Laboratory of Animal Science department at Uva Wellassa University for further analysis. All the eggs were cleaned and subjected to a storage experiment on open molded plastic egg trays at room temperature (25°C±2) to observe the variation trends of quality parameters with the storage time.
The Salmonella detection test was conducted on day 01 according to the protocol introduced by Roberts and Greenwood (2003). Five cleaned eggs [cleaned with 70% (v/v) ethanol, wiped, and left to dry at room temperature] were cracked and added into five conical flasks, each of which contained 180 mL of buffered peptone water. The mixtures were homogenized and incubated for 18 h at 37°C. Incubated media was cultured on Xylose Lysine Deoxycholate (XLD) agar (HiMedia Laboratories, Maharashtra, India). Cultured plates were incubated for 18 h at 37°C and examined for typical Salmonella colonies.
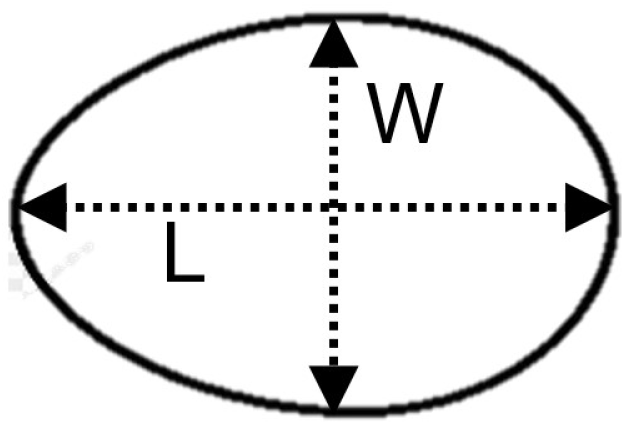
The shape index was measured based on the method suggested by Duman et al. (2016). The length (L) and width (W) of the egg were measured (Fig. 1) by using a Vernier caliper (147 6” digital caliper, General Tools and Instruments, Flusing, NY, USA). The following equation was used to calculate the shape index on day 01.
Eggs were graded according to the Egg Grading Manual of the United States Department of Agriculture (2000). The Haugh unit (HU) was calculated according to the method described by Stadelman and Cotterill (1995). Eggs were weighed, cracked, and poured gently onto the horizontal glass support. Albumen height (millimeter) was measured by using a spherometer (JD15160, Japson, Haryana, India) at predetermined time intervals.
Air cell depth was measured by using a Vernier caliper (147 6” digital caliper, General Tools and Instruments) and a candler (PI-01, P&I, Zhejiang, China). Accordingly, eggs were graded throughout the storage period on scheduled time intervals (Day 1, 3, 7, 10, 14, 21, 28, 35, 42, and 49) into AA, A, and B categories by considering the properties of the air cell, yolk, and albumen (Table 1).
| Quality factor | AAA | A | B |
|---|---|---|---|
| Air cell | |||
| Yolk | |||
| Albumen |
Egg weight loss percentage was measured according to Waimaleongora et al. (2009). The initial weight of the eggs was recorded on Day 1, and weight loss was computed on predefined time intervals (Days 3, 7, 10, 14, 21, 28, 35, 42, and 49) by using the following equation.
Yolk color was measured according to Sunder et al. (2022) by using a yolk color fan (A004, 2005-HMB, DSM, Switzerland). Yolk color was measured on established time intervals (Day 1, 3, 7, 10, 14, 21, 28, 35, 42, and 49) throughout the storage time.
The yolk index was measured as per the method used by Li et al. (2017). Yolk diameter was measured across the center of the yolk by using a Vernier caliper (147 6” digital caliper, General Tools and Instruments), and the height was measured by a spherometer (JD15160, Japson). The yolk index was calculated by using the below-mentioned equation. Measuring the parameter was done throughout the storage time.
Yolk and albumen percentages (Hristakieva et al., 2017) were calculated to identify the deterioration of eggs with the storage time. Initially, the weight of the whole egg was measured, and then the egg was cracked to separate the yolk by using an egg yolk separator. The weights of yolk and albumen were measured separately, and individual percentages were calculated.
The pH of the yolk and albumen were measured according to Pushpakumara et al. (2022) with some modifications. The yolk was separated from the albumen. Samples of yolk and albumen were prepared by adding 18 mL of distilled water to two grams of yolk and two grams of albumen separately. The samples were homogenized, and pH was measured by using a pH meter (PL-700PV, EZDO, Taipei City, Taiwan).
The shell thickness of the cracked eggs was measured according to Sun et al. (2012). Egg shells were rinsed, and membranes were removed. The thickness of the eggshell was measured by using an outside micrometer screw gauge (103-137, Mitutoyo, Kanagawa, Japan). Shell thickness was measured on Days 1, 14, 28, and 42.
Fourier Transform Infrared (FTIR) analysis was conducted according to Pushpakumara et al. (2022). The samples were prepared by freeze-drying egg albumin. A sample of 25 mg was used for the analysis by using an FT-IR machine (Alpha, Bruker, Germany). Results were obtained over the range of 500 cm−1 to 4,000 cm−1. Characterization of egg albumen and confirmation of distinct structures were done by curve fitting. The test was conducted on day 01 and 49.
The structure of hard-boiled eggs was observed according to the method described by Eregama et al. (2024). Eggs were boiled at 90°C for 15 min, peeled, and sliced so that the specimen was compatible with a microscopic view. The structure of egg albumen was examined under the power of ×80 by using gemological microscope (KSW8000, Kruss, Hamburg, Germany). The microscopic inspection test was done on Days 1, 28, and 60 of storage time.
3. Results and discussion
The negative pressure created due to temperature drop at the laying enhances bacterial penetration. In addition, the cuticle takes a short time to mature soon after the egg is laid, which may create a possibility for Salmonella to penetrate through the pores, contaminate, and multiply within the egg content. Besides, temperatures ranging from 20°C to 37°C are optimum growth temperatures for Salmonella enterica serovars (Chousalkar et al., 2021; Miyamoto et al., 1998). Therefore, storage of contaminated eggs due to horizontal or vertical transmission can cause the multiplication of Salmonella during the experiment (25°C±2). Hence, samples of eggs were tested for Salmonella with the motive of assessing quality and avoiding any unintentional breakout or multiplication during the storage and handling. All the tested samples were negative for the Salmonella test. Therefore, eggs were safely stored at room temperature (25°C±2) for the experiment with storage time. The experiment was conducted with predetermined time intervals of up to 49 days. According to the USDA (2024c), code dating can be used on cartons to add expiration dates, and the period cannot be more than 30 days from the day eggs were packed into the carton.
According to the egg grading manual by the USDA (2024a), shell shape is considered an external quality factor. A normal egg has an oval shape with a large end and tapering to form a smaller end. When grading the eggs, to be AA quality, there should be a normal oval shape and free from thin spots. In this experiment, the eggs were found to be of oval shape with an average shape index of 78.99±1.84. The shells were free from thin spots, ridges, and rough areas that can affect egg shape. Eggs were categorized into three based on shape index by Duman et al. (2016) as sharp (<72), normal (72-76), and round (>76) eggs. Accordingly, eggs in the experiment can be classified as round eggs. The shell index was measured at the initial stage to ensure that the eggs for the experiment were not misshaped.
The Haugh Unit (HU) is considered a measurement of the freshness of eggs that is influenced by environmental factors (temperature, time, and humidity of storage). A higher HU value indicates better albumin quality. However, thick albumin thins over storage time and converts to thin albumen, causing changes in pH and losing gel structure. This process affects the HU measurement (Kumari et al., 2022). The HU measurements, along with the visual parameters and air cell depth measurements described in Table 1, were used to grade eggs. In the current experiment, a significant decrease in HU (p<0.05) was identified (Fig. 2) with the storage time from Day 1 to 49 (98.77±2.67 to 39.44±4.95, respectively). According to the results, the ‘AA’ quality of eggs gradually loses its’ quality to ‘A’ on the third week (HU with 71.75±1.09, 66.93±2.83 on Days 21 and 28, respectively) and is observed to continue an ‘A’ grade on the fourth week. Further deterioration was examined, resulting in ‘B’ grade eggs with HU of 55.26±2.76 on Day 35. According to a study by Caner and Yuceer (2015), the HU of uncoated eggs was reduced to 58.93±0.96 on the fifth week of storage at 24°C.
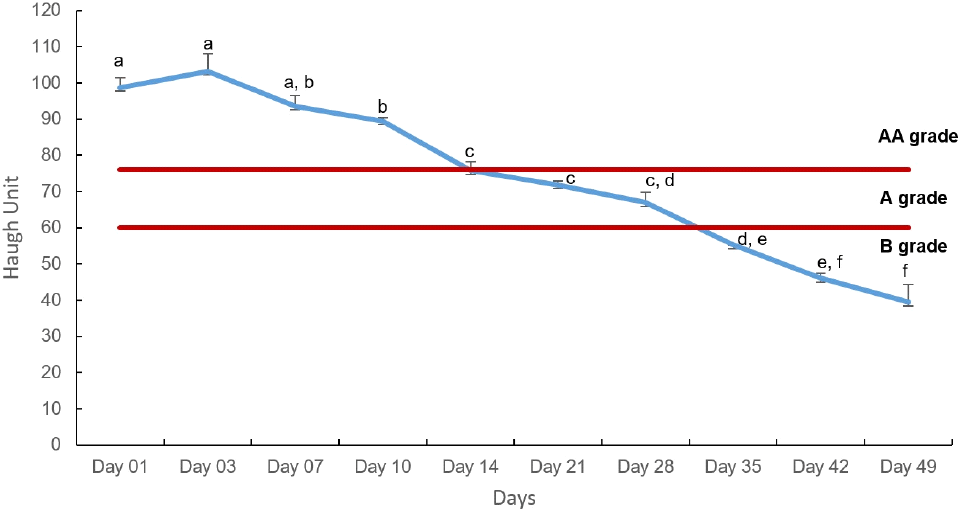
The USDA (2024b) states that ‘B’ grade eggs are often used for breaking stock and baking and must be concerned about defects in them during applications. Additionally, some sources state that consuming eggs whose HU is 70 is not recommended because of the loss of nutritional quality of eggs and the potential for microbial contamination (Martinez et al., 2021). Based on these findings, a conclusion can be drawn that shell eggs can be stored at room temperature to get ‘A’ grade eggs until 28 days of storage, which are suitable for direct consumption.
Egg weight is a quality parameter that decreases with increasing storage period due to the changes that occur, such as drying, shrinking of the cuticle, and increasing shell pore size, causing an exchange of carbon dioxide for oxygen and evaporation of moisture (Kumari et al., 2020). The initial average weight of the eggs was calculated and found to be 52.2 g. According to the weight class of US consumer grades for shell eggs, eggs used in the experiment can be categorized as medium size eggs (USDA, 2024a). A gradual increase in percentage weight loss was identified with the storage. In a previous study, where the storage of uncoated eggs at 24°C has shown similar results to the current study (Caner and Yuceer, 2015). The fifth-week reading for uncoated eggs stored at 24°C was recorded as 6.71%±0.73, while in the current study, 6.44%±0.87 on the 35th day. The grade was identified as ‘B,’ and the weight loss percentage was on Day 35 significantly different (p<0.05) from Days 21 and 28 (3.89%±0.45 and 5.04%±0.65, respectively), where the egg grade was A. Therefore, considering visual parameters, HU, and weight loss percentages (Table 2), the best storage period to get quality eggs at room temperature (25°C±2) can be decided as 28 days.
Yolk color, yolk index, pH, and percentages of albumen and yolk were considered during assessing the quality of the egg during the storage period (Table 3). During storage, carbonic acid in eggs breaks down into carbon dioxide and water. Carbon dioxide is expelled from egg pores, causing an increase in the pH of the egg white and losing the gel structure. Higher temperature causes higher loss of carbon dioxide and higher deterioration of the egg quality (Kumari et al., 2020). The albumen pH of a freshly laid egg lies between 7.6 and 9.7 (Quan et al., 2021). A previous study on eggs from Bovan White hens (50-week-old) showed that there is a significant increase in the pH of albumen on Day 10 of storage (9.11) compared to Day 1 (8.70) at 29°C (Samli et al., 2005). Similar results were observed in the current experiment, where the pH of albumen on Day 1, 8.06±0.20, was significantly increased to 9.14±0.12 on Day 7 (p<0.05). Thereafter, a significant difference in albumen pH was not identified until the end of the experiment on Day 49. Albumen pH was increased to 9.30±0.17 on Day 28, and no significant difference was identified when the quality of eggs changed from ‘AA’ grade eggs on Day 14 (9.20±0.09) to ‘A’ grade eggs on Day 28. In conclusion, other than visual parameters, HU value, and weight loss percentages, the deviation of albumen pH also suggests that eggs can be stored for 28 days at room temperature (25°C±2).
In the present research, the yolk index has significantly reduced (p<0.05) from 0.52±0.06 (Day 1) to 0.16±0.02 (Day 49) at room temperature. A significant reduction of yolk index is there between ‘AA’ graded eggs on Day 1 and ‘B’ graded eggs on Day 35 (0.23±0.03, p<0.05). A yolk index is a parameter that gives an idea about the shape of the yolk, whether it is round and firm or flattened. Egg yolks become flattened with storage mainly due to the entry of water via the vitelline membrane due to the breakdown of carbonic acid, and this causes an increase in permeability. The condition leads to egg yolk mottling (Kumari et al., 2020). Hence, in the current study, the shape of the yolk on Day 35 is considerably flattened compared to Day 1. The entering of water into the yolk can also be the reason for a significant increase in yolk weight percentage (from 25.42%±1.76 on Day 1 to 30.99%±0.55 on Day 49) and a significant decrease in albumen weight percentage (from 61.32%±1.24 on day 01 to 55.08%±0.84 on Day 49) with the storage period (p<0.05).
The yolk color of the egg is another quality parameter that is frequently measured by the yolk fan (A004, 2005-HMB, DSM). In the current study, a considerable change in yolk color was not identified with storage. The yolk color of eggs remained the same unit (10 in yolk color fan) throughout the storage period. Some studies (Martinez et al., 2021) recorded that there was a significant decrease in yolk color at the end of Day 10 (eggs stored at 26.50°C±5.48), where measurements were taken from an electronic colorimeter. However, according to the current study, there is no significant effect on yolk color when egg quality changes from ‘AA’ (Day 14) to ‘A’ grade (Days 21 and 28) when measured through the yolk color fan. This also facilitates the conclusion that eggs can be stored for 28 days at room temperature (25°C±2), considering most of the quality parameters.
According to previous studies by Grashorn et al. (2016), the magnitude of change of shell thickness was very small (in both 35-and 65-week-old categories of hens) with storage. In this experiment, shell thickness was measured with the storage since Stadelman and Cotterill (1995) describe that for eggs to have a better than 50% probability of surviving on typical market handling and to avoid cracking, the shell must be at least 0.33 mm thick. In the study, the eggshell thickness was significantly increased (p<0.05) on Day 28 (0.469±0.150 mm) compared to Day 1 (0.388±0.045 mm). Thus, shell thickness on Day 28 has a considerable probability of surviving on typical market handling.
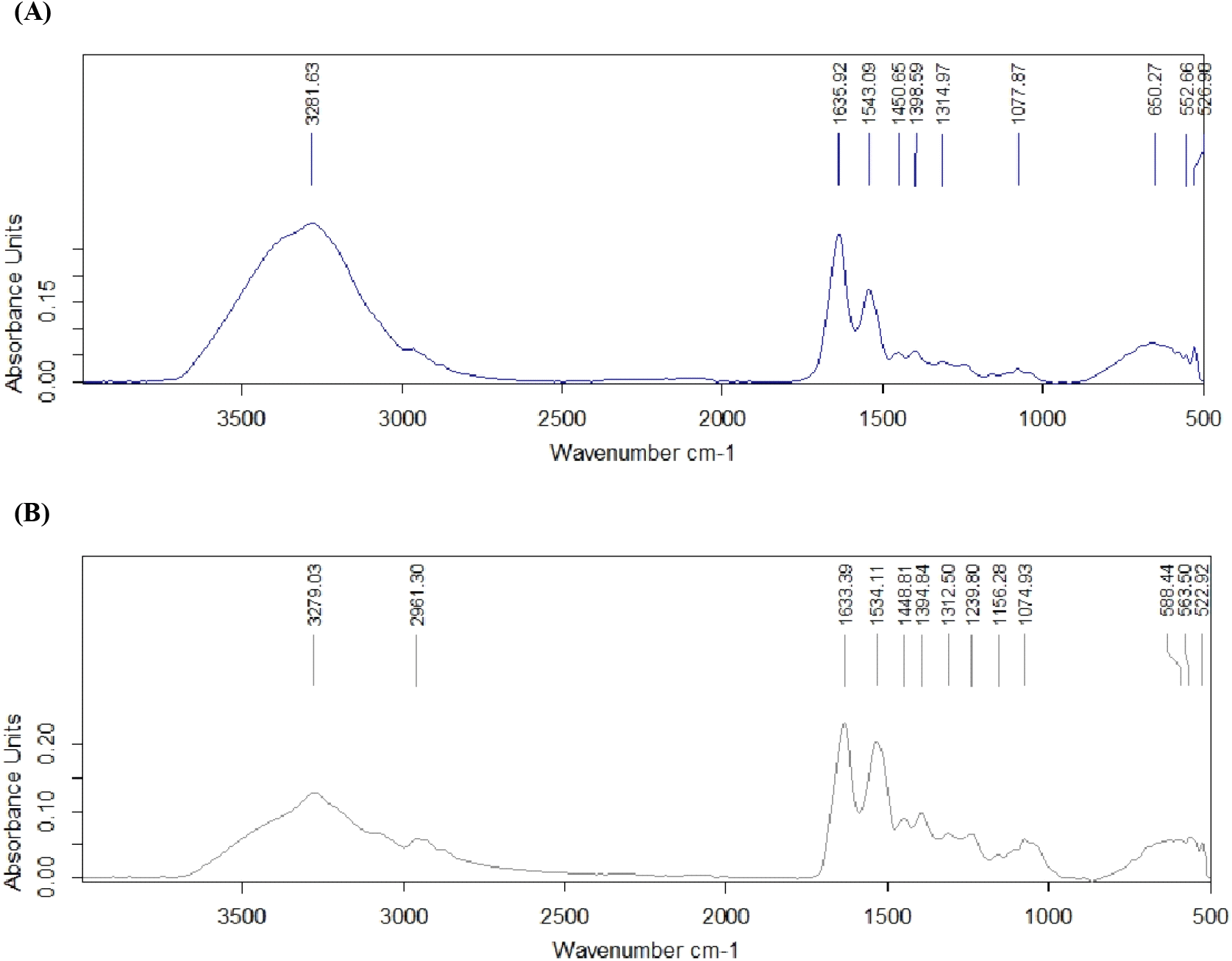
FTIR analysis was done to identify the deterioration of the egg composition with the storage period. The major difference identified between the eggs that are fresh and stored is in albumen pH and quality (Akter et al., 2014). Therefore, FTIR analysis was done for the egg albumen. Two main peaks (between 3,500-3,000 cm−1 and 2,000-1,500 cm−1) were obtained within the range of 400-4,000 cm−1 on both Day 1 (Fig. 3A) and Day 60 (Fig. 3B). A similar study by Eregama et al. (2024) helps to describe two peaks of the results. Accordingly, the peak at 3,282 cm−1 is due to the bond amide A of ovomucin (3,272.20 cm−1) and ovotransferrin (3,273.54 cm−1), peak at 1,636 cm−1 due to bond amide I (C=O stretch) of ovalbumin, lysozyme, ovomucoid, avidin, ovomucin, and ovotransferrin, while the peak at 1,543 cm−1 is due to amide II bond (N-H bend in the plane and C-N stretch) of lysozyme, avidin, ovomucin, and ovotransferrin. The deviation on the 60th day of storage on a peak between 3,500-3,000 cm−1 can be due to the reduction of disulfide bonds leading to the degradation of ovomucin or the disruption of the ovomucin-lysozyme complex, which has been identified as the possible reason for egg white thinning during extended storage (Guha et al., 2019).
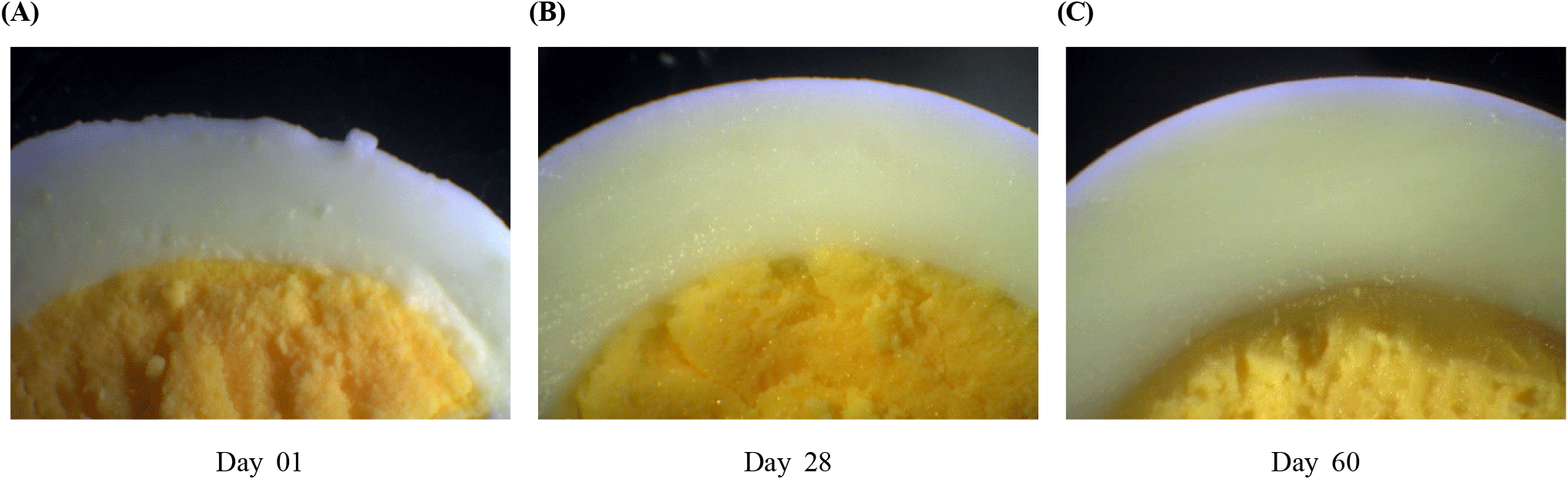
Boiling eggs is a simple and frequently used cooking method. Observing the hard-boiled structure of eggs under a gemological microscope is done to identify any change in structural integrity with storage that can affect the consumers’ perception. However, a clearly visible change in the structure of hard-boiled eggs was identified on Day 60 (Fig. 4) but not on Day 28. Despite the occurrence of significantly low HU, yolk index, and significantly highest loss of egg weight during storage (p<0.05), there was no visually visible difference identified in eggs stored at room temperature that were hard-boiled. Since a clear difference in the hard-boiled structure was not identified on Day 49, the storage period of the egg for this experiment was increased to 60 days to get a clear reference to show that even the hard-boiled structure is affected by the storage time. Therefore, limiting shelf life to 28 days will help consumers get additional benefits in terms of perception other than the quality parameters discussed above.
Sources record that most eggs also must be sold by the 21st and utilized by the 28th day of laying in the Chinese market (Huang et al., 2012). This experiment further supports the idea of previous studies where the shelf life of table eggs must be 28 days from laying under room temperature (25°C±2).
4. Conclusions
Eggs are frequently stored at room temperature in retail markets in developing countries. Furthermore, deterioration of egg quality occurs with storage conditions, which include temperature and time. Hence, for consumers and producers to consume and manufacture safe and high-quality food, it is very crucial to have a thorough understanding of the variation of quality parameters of eggs at ambient temperature. Finding variation trends in quality parameters of shell eggs that were stored at room temperature within the shelf life of the shell eggs was the primary goal of the study. In the current experiment, the sole focus was on the quality parameters that can be easily measured within the laboratory context to get an idea about how grading and other measurements change with the storage time. Therefore, after 28 days, deterioration of eggs occurred to B grade, which is usually considered good for breaking and baking stock as per the USDA egg-grading manual. Thus, after considering whole variation trends and data from gemological microscopy, it was concluded that poultry eggs can be stored for 28 days at room temperature (25°C), where their quality will remain at an ‘A’ grade, making them suitable for direct consumption. However, consumers must handle eggs carefully during storage as well. The extended next stage of the research is to investigate more microbial deterioration of the table eggs stored at ambient temperature (25°C) with storage time.










